A Novel Digital Care Management Platform to Monitor Clinical and Subclinical Disease Activity in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
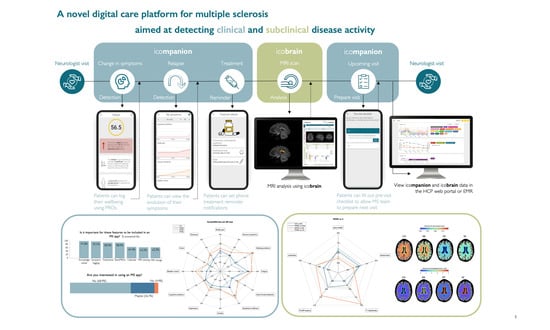
2.1. MS Care Management Platform
2.1.1. icompanion Patient App and Website
- The SymptoMScreen [21] is a 12-item battery for MS-related symptoms with a 7-point Likert scale per functional domain. Scores range from ‘not affected at all’ (score = 0) to ‘total limitation’ (score = 6). The SymptoMScreen composite is a score that summarizes general symptom severity by summing up the entered twelve symptom severities.
- The prEDSS is a patient-reported version of the Expanded Disability Status Scale (EDSS) which has shown to correlate well (Pearson’s coefficient 0.85) with a neurologist-scored EDSS [22].
- The Neuro-QoL (V1.0) Fatigue and (V2.0) Cognitive short-forms are short questionnaires consisting of eight items scored on a five-point scale each [23], and have been clinically validated in MS [19,20]. The Neuro-QoL t-scores reported in this paper are standardized scores with a mean of 50 and standard deviation (SD) of 10 compared to a reference population (for Cognitive a US general reference sample, for Fatigue a clinical US sample; http://www.healthmeasures.net/images/neuro_qol/Neuro_QOL_Scoring_Manual_Mar2015.pdf accessed on 27 August 2021). As an example, a t-score of 60 means that a person scores 1 SD higher than the reference sample.
2.1.2. icobrain ms Volumetric MRI Analyses
- top left = T1 overlaid with gray matter segmentation (blue) and T1 lesions (red);
- top middle = FLAIR overlaid with lesion segmentations color coded by location: periventricular (yellow), deep white matter (blue), juxtacortical (purple), and infratentorial (green);
- top right = FLAIR overlaid with color coded lesion changes compared to last available (or selected) scan: existing (green), enlarging (orange), new (red) lesions.
- bottom left = the quantitative report of whole brain and gray matter volume and atrophy (and comparison with healthy population).
- bottom middle = the quantitative report of existing, enlarging and, new FLAIR lesions and their location.
- bottom right = an example of a pre-populated radiological structured template, which already includes the icobrain ms measures and is available in the local language.
2.1.3. icompanion HCP Portal
2.2. Patient Perspective
2.2.1. Patient Survey 1: Telemonitoring Tools for Monitoring Clinical Disease Activity
2.2.2. Patient Survey 2: MR Imaging for Monitoring Subclinical Disease Activity
- ‘Would you like to view your MRI images on your own—Why or why not?’
- ‘If you have or had access to your own MRIs, would you be interested in knowing whether there were any changes between one MRI and the next (such as new lesions or loss of brain volume)?’
2.3. Monitoring Clinical Disease Activity Using icompanion: Real-World Evidence
2.3.1. Study Synopsis-Characterizing MS Types with icompanion
2.4. Monitoring Subclinical Disease Activity Using icobrain ms: Real-World Evidence
- -
- icobrain ms’ lesion annotations bring the performance of non-specialized radiologists closer to that of experienced neuroradiologists (Section 2.4.1)
- -
- the availability of icobrain ms reports in MS follow-up refine the assessment of subclinical disease activity (Section 2.4.2)
- -
- how the use of quantitative AI based brain MRI reporting can improve the radiological workflows (Section 2.4.2)
- -
- icobrain ms volumetric brain biomarkers bring insights into the brain patterns of MS types (Section 2.4.3)
2.4.1. Study Synopsis-Reliability of Lesion Count with icobrain ms
2.4.2. Study Synopsis-Detecting Subclinical Disease Activity in MRI Follow-Up with icobrain ms
2.4.3. Study Synopsis-Insights into the MS Brain Patterns Using icobrain ms
3. Results
3.1. Patient Perspective
3.1.1. Patient Survey 1: Telemonitoring Tools for Monitoring Clinical Disease Activity
3.1.2. Patient Survey 2: MR Imaging for Monitoring Subclinical Disease Activity
3.2. icompanion MS Patient App Validation
Sensitivity to Clinical Differences between MS Types
3.3. icobrain ms Brain MRI Analysis Validation
3.3.1. Reliability of Lesion Count with icobrain ms
3.3.2. Detecting Subclinical Disease Activity in MRI Follow-Up with icobrain ms
- stable: if they had no new or enlarging lesions and had normal rate of brain atrophy compared to controls (within 0.2% from normal atrophy rate of sex- and age-matched healthy controls in the case of icobrain ms measurements);
- slightly active: if they had enlarging lesions or slightly abnormal atrophy rate compared to controls (further than 0.2% but within 0.4% from normal atrophy rate of sex- and age-matched healthy controls in the case of icobrain ms measurements);
- active: if they had new lesions or severe progression of brain atrophy (further than 0.4% from the normal atrophy rate of sex- and age-matched healthy controls in the case of icobrain ms measurements).
3.3.3. Insights into the MS Brain Patterns: Sensitivity to Subclinical Differences between MS Types
4. Discussion
4.1. Patient Perspective
4.2. icompanion MS Patient App Validation
4.3. icobrain ms Brain MRI Analysis Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- What is your sex?
- ○
- Male
- ○
- Female
- ○
- Other
- What is your age?
- When were you diagnosed with MS?
- ○
- Less than 6 months ago
- ○
- 6 months–1 year ago
- ○
- 1–3 years ago
- ○
- 3–5 years ago
- ○
- 5–10 years ago
- ○
- 10–15 years ago
- ○
- 15–20 years ago
- ○
- Over 20 years ago
- ○
- I don’t know
- ○
- I prefer not to share this information
- Which type of MS do you have?
- ○
- Primary Progressive MS or PPMS
- ○
- Secondary Progressive MS or SPMS
- ○
- Relapsing Remitting MS or RRMS
- ○
- I don’t know
- ○
- I prefer not to share this information
- What is your attitude towards an MS app to help monitor your disease evolution?
- ○
- Very negative
- ○
- Rather negative
- ○
- Neutral
- ○
- Rather positive
- ○
- Very positive
- Is it important that a knowledge center is included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Is it important that symptom logging is included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Is it important that an overview and analysis of your MRI scans is included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Is it important that the evolution of your MRI scans is included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Is it important that tests are included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Is it important that a calendar to plan your doctor’s visits is included in an MS app?
- ○
- Yes
- ⯀
- Would you use this feature?
- Yes
- No
- ○
- No
- Are you interested in an MS app?
- ○
- Yes
- ○
- Maybe
- ○
- No
- How frequently would you use an MS app?
- ○
- Daily
- ○
- Multiple times per week
- ○
- Once per week
- ○
- Once every two weeks
- ○
- Once per month
- ○
- Other
Appendix B
- Have you ever had an MRI performed for the purpose of diagnosing or treating your MS?
- How often do you get an MRI for your MS, on average? Please disregard any recent delays in getting an MRI that you may have experienced due to the COVID-19 pandemic.
- When did you last get an MRI of your brain?
- When did you last get an MRI of your neck or spinal column?
- Have you ever received or been given access to an electronic copy of your MRI? Check all that apply.
- ○
- Yes, my clinic or radiology lab gave it to me without my requesting it
- ○
- Yes, my clinic or radiology lab gave it to me after I requested it
- ○
- Yes, I got one from participating in a research study
- ○
- No
- ○
- Not sure/prefer not to say
- Have you ever received or been given access to an electronic copy of your MRI? Check all that apply.
- ○
- CD/disc
- ○
- USB drive/thumb drive
- ○
- Direct download into my computer or other device
- ○
- Access through my clinic’s patient portal
- ○
- Other—Write In
- ○
- Not sure/prefer not to say
- Have you ever looked at your own MRI images on your own, without your healthcare provider present?
- ○
- [in case of Yes] Did you find it helpful in any way to view your MRI images on your own? If so, how?
- ○
- [in case of Yes] Did you find it unhelpful in any way to view your MRI images on your own? If so, how?
- ○
- [in case of Yes] How well did you understand the images on your MRI?
- ○
- [in case of No] Why haven’t you looked at your MRI images on your own? Check all that apply.
- ⯀
- I wasn’t sure how to view the images
- ⯀
- I didn’t have a software program or application for viewing the files
- ⯀
- I couldn’t load the images onto my computer or another device where I could view them
- ⯀
- I didn’t know how to interpret the images
- ⯀
- I wasn’t interested in viewing my MRI images on my own
- ⯀
- Not sure/prefer not to say:
- ⯀
- Other—Write In
- ○
- [in case of No] Would you like to view your MRI images on your own? Why or why not?
- Would you be interested in having your own copy of your MRI images to view?Why or why not?
- If you have or had access to your own MRIs, would you be interested in knowing whether the MRI was performed according to the current clinical guidelines for MS? Why or why not?
- If you have or had access to your own MRIs, would you be interested in knowing whether there were any changes between one MRI and the next (such as new lesions or loss of brain volume)? Why or why not?
- If you have or had access to your own MRIs, would you be willing to share your electronic MRI with a researcher, if asked? Why or why not?
- If you have or had access to your own MRIs, would you be willing to share them with iConquerMS, if asked, and allow iConquerMS to share them with researchers? Why or why not?
References
- Coetzee, T.; Thompson, A.J. Atlas of MS 2020: Informing Global Policy Change. Mult. Scler. 2020, 26, 1807–1808. [Google Scholar] [CrossRef]
- National MS Society (Medications). Available online: https://www.nationalmssociety.org/Treating-MS/Medications (accessed on 29 July 2021).
- Hult, K. Measuring the Potential Health Impact of Personalized Medicine: Evidence from MS Treatments; National Bureau of Economic Research, Inc.: Cambridge, MA, USA, 2017. [Google Scholar]
- Sá, M.J.; de Sá, J.; Sousa, L. Relapsing–Remitting Multiple Sclerosis: Patterns of Response to Disease-Modifying Therapies and Associated Factors: A National Survey. Neurol. Ther. 2014, 3, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, K.K.; Butler, J.S.; Mattingly, M.; Ryan, M. Factors Leading Patients to Discontinue Multiple Sclerosis Therapies. J. Am. Pharm. Assoc. 2005, 45, 371–375. [Google Scholar] [CrossRef]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain Health: Time Matters in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2016, 9 (Suppl. 1), S5–S48. [Google Scholar] [CrossRef] [Green Version]
- Duddy, M.; Lee, M.; Pearson, O.; Nikfekr, E.; Chaudhuri, A.; Percival, F.; Roberts, M.; Whitlock, C. The UK Patient Experience of Relapse in Multiple Sclerosis Treated with First Disease Modifying Therapies. Mult. Scler. Relat. Disord. 2014, 3, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.P.; Ponziani, G.; Siracusa, G.; Sorbi, S. Cognitive Dysfunction in Early-Onset Multiple Sclerosis: A Reappraisal after 10 Years. Arch. Neurol. 2001, 58, 1602–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnano, I.; Aiello, I.; Piras, M.R. Cognitive Impairment and Neurophysiological Correlates in MS. J. Neurol. Sci. 2006, 245, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kürtüncü, M.; Tuncer, A.; Uygunoğlu, U.; Çalişkan, Z.; Paksoy, A.K.; Efendı, H.; Kocaman, A.S.; Özcan, C.; Terzı, M.; Turan, Ö.F.; et al. Differences Between General Neurologists and Multiple Sclerosis Specialists in the Management of Multiple Sclerosis Patients: A National Survey. Noro psikiyatri arsivi 2017, 56. [Google Scholar] [CrossRef] [Green Version]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS–CMSC–NAIMS Consensus Recommendations on the Use of MRI in Patients with Multiple Sclerosis. Lancet Neurol. 2021. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Duszak, R.; Babb, J.S.; Glover, M.; Kang, S.K. Discrepancy Rates and Clinical Impact of Imaging Secondary Interpretations: A Systematic Review and Meta-Analysis. J. Am. Coll. Radiol. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Voigt, I.; Scholz, M.; Schlieter, H.; Benedict, M.; Susky, M.; Dillenseger, A.; Ziemssen, T. Profiles of eHealth Adoption in Persons with Multiple Sclerosis and Their Caregivers. Brain Sciences 2021, 11, 1087. [Google Scholar] [CrossRef]
- Ziemssen, T.; Thomas, K. Treatment Optimization in Multiple Sclerosis: How Do We Apply Emerging Evidence? Expert Rev. Clin. Immunol. 2017, 13, 509–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, E.; Haase, R.; Ziemssen, T. Review: Patient-Reported Outcomes in Multiple Sclerosis Care. Mult. Scler. Relat. Disord. 2019, 33, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Celius, E.G.; Thompson, H.; Pontaga, M.; Langdon, D.; Laroni, A.; Potra, S.; Bharadia, T.; Yeandle, D.; Shanahan, J.; van Galen, P.; et al. Disease Progression in Multiple Sclerosis: A Literature Review Exploring Patient Perspectives. Patient Prefer. Adherence 2021, 15, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Neuner, B.; Kasper, J.; Vodermaier, A.; Loh, A.; Deinzer, A.; Heesen, C.; Kissling, W.; Busch, R.; Schmieder, R.; et al. Participation Preferences of Patients with Acute and Chronic Conditions. Health Expect. 2007, 10, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leeuwen, K.G.; Schalekamp, S.; Mjcm, R.; van Ginneken, B.; de Rooij, M. Artificial Intelligence in Radiology: 100 Commercially Available Products and Their Scientific Evidence. Eur. Radiol. 2021, 31. [Google Scholar] [CrossRef]
- Medina, L.D.; Torres, S.; Alvarez, E.; Valdez, B.; Nair, K.V. Patient-Reported Outcomes in Multiple Sclerosis: Validation of the Quality of Life in Neurological Disorders (Neuro-QoLTM) Short Forms. Mult Scler J Exp Transl Clin 2019, 5, 2055217319885986. [Google Scholar] [CrossRef]
- Miller, D.M.; Bethoux, F.; Victorson, D.; Nowinski, C.J.; Buono, S.; Lai, J.-S.; Wortman, K.; Burns, J.L.; Moy, C.; Cella, D. Validating Neuro-QoL Short Forms and Targeted Scales with People Who Have Multiple Sclerosis. Mult. Scler. 2016, 22, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Green, R.; Kalina, J.; Ford, R.; Pandey, K.; Kister, I. SymptoMScreen: A Tool for Rapid Assessment of Symptom Severity in MS Across Multiple Domains. Appl. Neuropsychol. Adult 2017, 24, 183–189. [Google Scholar] [CrossRef]
- Leddy, S.; Hadavi, S.; McCarren, A.; Giovannoni, G.; Dobson, R. Validating a Novel Web-Based Method to Capture Disease Progression Outcomes in Multiple Sclerosis. J. Neurol. 2013, 260, 2505–2510. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.-S.; Nowinski, C.J.; Victorson, D.; Peterman, A.; Miller, D.; Bethoux, F.; Heinemann, A.; Rubin, S.; Cavazos, J.E.; et al. Neuro-QOL: Brief Measures of Health-Related Quality of Life for Clinical Research in Neurology. Neurology 2012, 78, 1860–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Sima, D.M.; Ribbens, A.; Cambron, M.; Maertens, A.; Van Hecke, W.; De Mey, J.; Barkhof, F.; Steenwijk, M.D.; Daams, M.; et al. Automatic Segmentation and Volumetry of Multiple Sclerosis Brain Lesions from MR Images. NeuroImage Clin. 2015, 8, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Rakić, M.; Vercruyssen, S.; Van Eyndhoven, S.; de la Rosa, E.; Jain, S.; Van Huffel, S.; Maes, F.; Smeets, D.; Sima, D.M. Icobrain Ms 5.1: Combining Unsupervised and Supervised Approaches for Improving the Detection of Multiple Sclerosis Lesions. Neuroimage Clin 2021, 31, 102707. [Google Scholar] [CrossRef]
- Smeets, D.; Ribbens, A.; Sima, D.M.; Cambron, M.; Horakova, D.; Jain, S.; Maertens, A.; Van Vlierberghe, E.; Terzopoulos, V.; Van Binst, A.-M.; et al. Reliable Measurements of Brain Atrophy in Individual Patients with Multiple Sclerosis. Brain Behav. 2016, 6, e00518. [Google Scholar] [CrossRef] [PubMed]
- Lysandropoulos, A.P.; Absil, J.; Metens, T.; Mavroudakis, N.; Guisset, F.; Van Vlierberghe, E.; Smeets, D.; David, P.; Maertens, A.; Van Hecke, W. Quantifying Brain Volumes for Multiple Sclerosis Patients Follow-up in Clinical Practice—Comparison of 1.5 and 3 Tesla Magnetic Resonance Imaging. Brain Behav. 2016, 6, e00422. [Google Scholar] [CrossRef] [PubMed]
- Beadnall, H.N.; Wang, C.; Van Hecke, W.; Ribbens, A.; Billiet, T.; Barnett, M.H. Comparing Longitudinal Brain Atrophy Measurement Techniques in a Real-World Multiple Sclerosis Clinical Practice Cohort: Towards Clinical Integration? Ther. Adv. Neurol. Disord. 2019, 12, 175628641882346. [Google Scholar] [CrossRef]
- Costers, L.; Schmidt, H.; Loud, S.; McBurney, R.; Gwynne, D.; Van Vlierberghe, E.; Descamps, A.; Smeets, D.; Van Hecke, W. MRI in MS Survey—Insights into Access, Understanding and Interest by People with MS. Mult. Scler. J. 2021, 27, 70. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kozlowski, A.J.; Cella, D.; Nitsch, K.P.; Heinemann, A.W. Evaluating Individual Change with the Quality of Life in Neurological Disorders (Neuro-QoL) Short Forms. Arch. Phys. Med. Rehabil. 2016, 97, 650–654.e8. [Google Scholar] [CrossRef] [Green Version]
- Ion-Mărgineanu, A.; Kocevar, G.; Stamile, C.; Sima, D.M.; Durand-Dubief, F.; Van Huffel, S.; Sappey-Marinier, D. Machine Learning Approach for Classifying Multiple Sclerosis Courses by Combining Clinical Data with Lesion Loads and Magnetic Resonance Metabolic Features. Front. Neurosci. 2017, 11, 398. [Google Scholar] [CrossRef]
- Sima, D.M.; Podevyn, F.; Torcida, N.; Wilms, G.; Lysandropoulos, A.; Van Hecke, W.; Smeets, D. Impact of MSmetrix Automatic Lesion Segmentation on the Visual Count of Multiple Sclerosis Lesions. In Proceedings of the 2018 European Congress of Radiology, Vienna, Austria, 28 February–4 March 2018; p. C-2472. [Google Scholar]
- Sima, D.M.; Wilms, G.; Vyvere, T.V.; Van Hecke, W.; Smeets, D. On the Use of Icobrain’s Prepopulated Radiology Reporting Template for Multiple Sclerosis Follow-Up. In Proceedings of the 2020 European Congress of Radiology, Vienna, Austria, 15–19 July 2020; p. C-11342. [Google Scholar]
- Sima, D.M.; Jain, S.; Roura, E.; Maertens, A.; Smeets, D.; Sappey-Marinier, D.; Durand-Dubief, F.; Van Hecke, W. New and Enlarging Lesion Location for Different MS Clinical Phenotypes. In Proceedings of the MSParis2017—7th Joint ECTRIMS-ACTRIMS, Paris, France, 25–28 October 2017; p. EP1559. [Google Scholar]
- Potemkowski, A.; Brola, W.; Ratajczak, A.; Ratajczak, M.; Zaborski, J.; Jasińska, E.; Pokryszko-Dragan, A.; Gruszka, E.; Dubik-Jezierzańska, M.; Podlecka-Piętowska, A.; et al. Internet Usage by Polish Patients with Multiple Sclerosis: A Multicenter Questionnaire Study. Interact. J. Med. Res. 2019, 8, e11146. [Google Scholar] [CrossRef]
- Brand, J.; Köpke, S.; Kasper, J.; Rahn, A.; Backhus, I.; Poettgen, J.; Stellmann, J.-P.; Siemonsen, S.; Heesen, C. Magnetic Resonance Imaging in Multiple Sclerosis--Patients’ Experiences, Information Interests and Responses to an Education Programme. PLoS ONE 2014, 9, e113252. [Google Scholar] [CrossRef]
- Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Severo, M.; Patti, F.; Cilia, S.; Gallo, P.; Grossi, P.; Ghezzi, A.; et al. Age and Disability Drive Cognitive Impairment in Multiple Sclerosis across Disease Subtypes. Mult. Scler. 2017, 23, 1258–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijeholt, G.J.; van Walderveen, M.A.; Castelijns, J.A.; van Waesberghe, J.H.; Polman, C.; Scheltens, P.; Rosier, P.F.; Jongen, P.J.; Barkhof, F. Brain and Spinal Cord Abnormalities in Multiple Sclerosis. Correlation between MRI Parameters, Clinical Subtypes and Symptoms. Brain 1998, 121 Pt 4, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercruyssen, S.; Brys, A.; Verheijen, M.; Steach, B.; Van Vlierberghe, E.; Sima, D.M.; Smeets, D. Conformance to CMSC Magnetic Resonance Imaging (MRI) Guidelines in a Real-World Multicenter MRI Dataset. Int. J. MS Care 2020, 22, 47. [Google Scholar]
- Tsagkas, C.; Magon, S.; Gaetano, L.; Pezold, S.; Naegelin, Y.; Amann, M.; Stippich, C.; Cattin, P.; Wuerfel, J.; Bieri, O.; et al. Preferential Spinal Cord Volume Loss in Primary Progressive Multiple Sclerosis. Mult. Scler. J. 2019, 25, 947–957. [Google Scholar] [CrossRef]
- Dahan, A.; Wang, W.; Gaillard, F. Computer-Aided Detection Can Bridge the Skill Gap in Multiple Sclerosis Monitoring. J. Am. Coll. Radiol. 2018, 15, 93–96. [Google Scholar] [CrossRef]
- Wang, W.; van Heerden, J.; Tacey, M.A.; Gaillard, F. Neuroradiologists Compared with Non-Neuroradiologists in the Detection of New Multiple Sclerosis Plaques. AJNR Am. J. Neuroradiol. 2017, 38, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- van Heerden, J.; Rawlinson, D.; Zhang, A.M.; Chakravorty, R.; Tacey, M.A.; Desmond, P.M.; Gaillard, F. Improving Multiple Sclerosis Plaque Detection Using a Semiautomated Assistive Approach. AJNR Am. J. Neuroradiol. 2015, 36, 1465–1471. [Google Scholar] [CrossRef] [Green Version]
- Zopfs, D.; Laukamp, K.R.; Paquet, S.; Lennartz, S.; Pinto Dos Santos, D.; Kabbasch, C.; Bunck, A.; Schlamann, M.; Borggrefe, J. Follow-up MRI in Multiple Sclerosis Patients: Automated Co-Registration and Lesion Color-Coding Improves Diagnostic Accuracy and Reduces Reading Time. Eur. Radiol. 2019, 29, 7047–7054. [Google Scholar] [CrossRef]
- Beadnall, H.N.; Ly, L.; Wang, C.; Billiet, T.; Ribbens, A.; Van Hecke, W.; Zivadinov, R.; Barnett, M.H. 103 Exploring the Influence of Quantitative Magnetic Resonance Imaging on Decision-Making in Multiple Sclerosis Clinical Practice. J. Neurol. Neurosurg. Psychiatry 2018, 89, A41. [Google Scholar]
- Lexa, F.J. Duty Hour Limits for Radiologists: It’s About Time. J. Am. Coll. Radiol. 2021, 18, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Natural History of Multiple Sclerosis: A Unifying Concept. Brain 2006, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| CIS 3.1% n = 42 | RRMS 78.4% n = 1061 | SPMS 10.9% n = 147 | PPMS 7.6% n = 103 | Group Effect | |
|---|---|---|---|---|---|
| Gender (female) | 76.2% | 75.7% | 61.2% | 63.1% | |
| Age | 33.9 (10.2, 41) | 39.7 (10.4, 1058) | 47.7 (12.6, 144) | 45.8 (12.6, 101) | |
| Disease duration | 5.24 (6.87, 42) | 7.98 (6.84, 1061) | 16.5 (9.21, 147) | 7.38 (6.25, 103) | |
| Mental feeling | 0.17 (1.17, 33) | 0.49 (0.91, 869) | 0.37 (0.89, 114) | 0.47 (0.91, 81) | H(3) = 5.15 p = 0.193 |
| Physical feeling | 0.12 (0.89, 33) | 0.25 (0.88, 862) | −0.05 (0.81, 113) | 0.06 (0.85, 80) | H(3) = 10.85 p = 0.025 |
| prEDSS | 2.87 (1.77, 16) | 3.35 (1.57, 336) | 5.13 (1.38, 41) | 5.02 (1.62, 31) | H(3) = 64.47 p < 0.001 |
| Sympto-MScreen composite | 10.5 (13.2, 40) | 12.8 (13.5, 1024) | 17.0 (15.5, 140) | 17.9 (15.9, 101) | H(3) = 15.40 p = 0.005 |
| Neuro-QoL Fatigue | 54.0 (11.91, 15) | 55.1 (8.75, 319) | 56.0 (7.74, 38) | 55.1 (8.95, 30) | H(3) = 0.31 p = 0.312 |
| Neuro-QoL Cognitive | 48.5 (8.95, 15) | 43.7 (8.87, 334) | 42.0 (9.09, 41) | 44.2 (7.97, 30) | H(3) = 5.22 p = 0.193 |
| Whole Brain | Gray Matter | Lateral Ventricles | FLAIR Lesions | T1 Blackholes | |
|---|---|---|---|---|---|
| EDSS ≤ 4 | |||||
| Group effect | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| CIS vs. RRMS | p = 0.008 | p = 0.258 | p = 0.029 | p < 0.001 | p < 0.001 |
| CIS vs. SPMS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| CIS vs. PPMS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| RRMS vs. SPMS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| RRMS vs. PPMS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.173 |
| SPMS vs. PPMS | p = 0.935 | p = 1.000 | p = 1.000 | p = 0.902 | p < 0.001 |
| EDSS > 4 | |||||
| Group effect | p = 0.013 | p = 0.072 | p = 0.014 | p = 0.013 | p = 0.054 |
| RRMS vs. SPMS | p = 0.005 | p = 0.073 | p = 0.006 | p = 0.034 | p = 0.101 |
| RRMS vs. PPMS | p = 0.007 | p = 0.094 | p = 0.027 | p = 0.716 | p = 0.662 |
| SPMS vs. PPMS | p = 0.992 | p = 1.000 | p = 0.955 | p = 0.033 | p = 0.251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hecke, W.; Costers, L.; Descamps, A.; Ribbens, A.; Nagels, G.; Smeets, D.; Sima, D.M. A Novel Digital Care Management Platform to Monitor Clinical and Subclinical Disease Activity in Multiple Sclerosis. Brain Sci. 2021, 11, 1171. https://doi.org/10.3390/brainsci11091171
Van Hecke W, Costers L, Descamps A, Ribbens A, Nagels G, Smeets D, Sima DM. A Novel Digital Care Management Platform to Monitor Clinical and Subclinical Disease Activity in Multiple Sclerosis. Brain Sciences. 2021; 11(9):1171. https://doi.org/10.3390/brainsci11091171
Chicago/Turabian StyleVan Hecke, Wim, Lars Costers, Annabel Descamps, Annemie Ribbens, Guy Nagels, Dirk Smeets, and Diana M. Sima. 2021. "A Novel Digital Care Management Platform to Monitor Clinical and Subclinical Disease Activity in Multiple Sclerosis" Brain Sciences 11, no. 9: 1171. https://doi.org/10.3390/brainsci11091171