fMRI Investigation of Semantic Lexical Processing in Healthy Control and Alzheimer’s Disease Subjects Using Naming Task: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
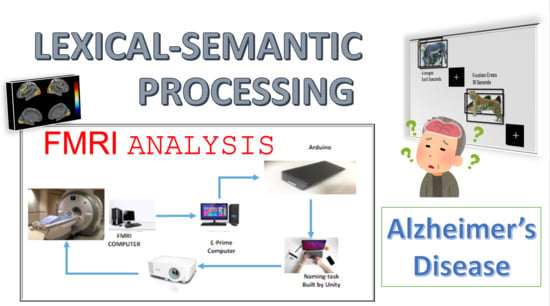
2.2. Protocols and Materials
2.3. FMRI Data Acquisition, Preprocessing, and Statistical Analysis
3. Results
3.1. Healthy Controls
3.1.1. Whole Brain Analysis
3.1.2. Region of Interest (ROI) Analysis
3.2. Alzheimers Disease
3.2.1. Whole Brain Analysis
3.2.2. Region of Interest (ROI) Analysis
3.3. Group Comparison
3.3.1. Whole Brain Analysis
3.3.2. Region of Interest (ROI) Analysis
4. Discussion
4.1. Healthy Control Group
4.2. Alzheimers Disease Group
4.3. Inconsistent Pattern of Brain Activity
4.4. Other Active Regions
5. Study Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Alzheimer Report 2020—Design Dignity Dementia: Dementia-Related Design and the Built Environment; Alzheimers Disease International: London, UK, 2020; Volume 1, p. 248.
- Salmon, D.P.; Butters, N.; Chan, A.S. The deterioration of semantic memory in Alzheimer’s disease. Can. J. Exp. Psychol. 1999, 53, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chertkow, H.; Bub, D. Semantic memory loss in dementia of Alzheimer’s type. What do various measures measure? Brain 1990, 113 Pt 2, 397–417. [Google Scholar] [CrossRef]
- WHODementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 15 January 2021).
- Chen, Y.T.; Hou, C.J.; Derek, N.; Huang, S.B.; Huang, M.W.; Wang, Y.Y. Evaluation of the Reaction Time and Accuracy Rate in Normal Subjects, MCI, and Dementia Using Serious Games. Appl. Sci. 2021, 11, 628. [Google Scholar] [CrossRef]
- Bayram, E.; Caldwell, J.Z.K.; Banks, S.J. Current understanding of magnetic resonance imaging biomarkers and memory in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Howard, R.J. Semantic memory and language dysfunction in early Alzheimer’s disease: A review. Int. J. Geriatr. Psychiatry 2012, 27, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb. Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef] [PubMed]
- Atri, A.; O’Brien, J.L.; Sreenivasan, A.; Rastegar, S.; Salisbury, S.; DeLuca, A.N.; O’Keefe, K.M.; LaViolette, P.S.; Rentz, D.M.; Locascio, J.J.; et al. Test-retest reliability of memory task functional magnetic resonance imaging in Alzheimer disease clinical trials. Arch. Neurol. 2011, 68, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Stricker, N.H.; McCauley, A.; Simmons, A.; Jak, A.J.; Chang, Y.L.; Nation, D.A.; Bangen, K.J.; Salmon, D.P.; Bondi, M.W. Altered brain response for semantic knowledge in Alzheimer’s disease. Neuropsychologia 2011, 49, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Hodges, J.R.; Salmon, D.P.; Butters, N. The nature of the naming deficit in Alzheimer’s and Huntington’s disease. Brain 1911, 114 Pt 4, 1547–1558. [Google Scholar] [CrossRef]
- Frank, E.M.; McDade, H.L.; Scott, W.K. Naming in dementia secondary to Parkinson’s, Huntington’s, and Alzheimer’s diseases. J. Commun. Disord. 1996, 29, 183–197. [Google Scholar] [CrossRef]
- Balthazar, M.L.; Yasuda, C.L.; Lopes, T.M.; Pereira, F.R.; Damasceno, B.P.; Cendes, F. Neural correlates of lexical-semantic memory: A voxel-based morphometry study in mild AD, aMCI and normal aging. Dement. Neuropsychol. 2011, 5, 69–77. [Google Scholar] [CrossRef]
- Joubert, S.; Brambati, S.M.; Ansado, J.; Barbeau, E.J.; Felician, O.; Didic, M.; Lacombe, J.; Goldstein, R.; Chayer, C.; Kergoat, M.J. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 2010, 48, 978–988. [Google Scholar] [CrossRef]
- Mickes, L.; Wixted, J.T.; Fennema-Notestine, C.; Galasko, D.; Bondi, M.W.; Thal, L.J.; Salmon, D.P. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology 2007, 21, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Chao, L.L. Semantic memory and the brain: Structure and processes. Curr. Opin. Neurobiol. 2001, 11, 194–201. [Google Scholar] [CrossRef]
- Chouinard, P.A.; Goodale, M.A. Category-specific neural processing for naming pictures of animals and naming pictures of tools: An ALE meta-analysis. Neuropsychologia 2010, 48, 409–418. [Google Scholar] [CrossRef]
- Garrard, P.; Lambon Ralph, M.A.; Patterson, K.; Pratt, K.H.; Hodges, J.R. Semantic feature knowledge and picture naming in dementia of Alzheimer’s type: A new approach. Brain Lang. 2005, 93, 79–94. [Google Scholar] [CrossRef]
- Hodges, J.R.; Salmon, D.P.; Butters, N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia 1992, 30, 301–314. [Google Scholar] [CrossRef]
- Vogel, A.; Gade, A.; Stokholm, J.; Waldemar, G. Semantic Memory Impairment in the Earliest Phases of Alzheimers Disease; Karger Publishers: Basel, Switzerland, 2005; Volume 19, pp. 75–81. [Google Scholar] [CrossRef]
- Guidi, M.; Paciaroni, L.; Paolini, S.; Scarpino, O.; Burn, D.J. Semantic profiles in mild cognitive impairment associated with Alzheimer’s and Parkinson’s diseases. Funct. Neurol. 2015, 30, 113–118. [Google Scholar] [CrossRef]
- Taler, V.; Phillips, N.A. Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. J. Clin. Exp. Neuropsychol. 2008, 30, 501–556. [Google Scholar] [CrossRef]
- Bayles, K.A.; Tomoeda, C.K. Confrontation naming impairment in dementia. Brain Lang. 1983, 19, 98–114. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Stricker, N.H.; McCauley, A.; Simmons, A.; Jak, A.J.; Chang, Y.L.; Delano-Wood, L.; Bangen, K.J.; Salmon, D.P.; Bondi, M.W. Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer’s disease. Neuroimage 2010, 51, 1222–1233. [Google Scholar] [CrossRef] [Green Version]
- Paek, E.J.; Murray, L.L.; Newman, S.D.; Kim, D.J. Test-retest reliability in an fMRI study of naming in dementia. Brain Lang. 2019, 191, 31–45. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Perlstein, W.M.; Benjamin, M.; Leonard, C.M.; Rothi, L.G.; Conway, T.; Cato, M.A.; Gopinath, K.; Briggs, R.; Crosson, B. Neural substrates of object identification: Functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. J. Int. Neuropsychol. Soc. 2009, 15, 169–181. [Google Scholar] [CrossRef]
- Anderson, A.J.; Lin, F. How pattern information analyses of semantic brain activity elicited in language comprehension could contribute to the early identification of Alzheimer’s Disease. Neuroimage Clin. 2019, 22, 101788. [Google Scholar] [CrossRef]
- Paek, E.J.; Murray, L.L.; Newman, S.D. Neural Correlates of Verb Fluency Performance in Cognitively Healthy Older Adults and Individuals With Dementia: A Pilot fMRI Study; Frontiers: Lausanne, Switzerland, 2020; Volume 12. [Google Scholar] [CrossRef] [Green Version]
- Reilly, J.; Peelle, J.E.; Antonucci, S.M.; Grossman, M. Anomia as a Marker of Distinct Semantic Memory Impairments in Alzheimers Disease and Semantic Dementia. Neuropsychology 2011, 25, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.S. fMRI for mapping language networks in neurosurgical cases. Indian J. Radiol. Imaging 2014, 24, 37–43. [Google Scholar] [CrossRef]
- Gesierich, B.; Jovicich, J.; Riello, M.; Adriani, M.; Monti, A.; Brentari, V.; Robinson, S.D.; Wilson, S.M.; Fairhall, S.L.; Gorno-Tempini, M.L. Distinct Neural Substrates for Semantic Knowledge and Naming in the Temporoparietal Network. Cereb. Cortex 2012, 22, 2217–2226. [Google Scholar] [CrossRef] [Green Version]
- Domoto-Reilly, K.; Sapolsky, D.; Brickhouse, M.; Dickerson, B.C.; Alzheimer’s Disease Neuroimaging Initiative. Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. Neuroimage 2012, 63, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Melrose, R.J.; Campa, O.M.; Harwood, D.G.; Osato, S.; Mandelkern, M.A.; Sultzer, D.L. The neural correlates of naming and fluency deficits in Alzheimers disease: An FDG-PET study. Int. J. Geriatr. Psychiatry A J. Psychiatry Late Life Allied Sci. 2009, 24, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, S.; McMahon, K.L.; Nickels, L.A.; Angwin, A.; MacDonald, A.D.; van Hees, S.; McKinnon, E.; Johnson, K.; Copland, D.A. An fMRI investigation of the effects of attempted naming on word retrieval in aphasia. Front. Hum. Neurosci. 2015, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.L.; Booth, J.R.; Burman, D.D.; Bitan, T.; Bigio, J.D.; Lu, D.; Cone, N.E. Developmental changes in the neural correlates of semantic processing. Neuroimage 2006, 29, 1141–1149. [Google Scholar] [CrossRef]
- Russo, N.; Hagmann, C.E.; Andrews, R.; Black, C.; Silberman, M.; Shea, N. Validation of the C.A.R.E. stimulus set of 640 animal pictures: Name agreement and quality ratings. PLoS ONE 2018, 13, e0192906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devlin, J.T.; Russell, R.P.; Davis, M.H.; Price, C.J.; Moss, H.E.; Fadili, M.J.; Tyler, L.K. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia 2002, 40, 54–75. [Google Scholar] [CrossRef]
- Saykin, A.J.; Flashman, L.A.; Frutiger, S.A.; Johnson, S.C.; Mamourian, A.C.; Moritz, C.H.; OJile, J.R.; Riordan, H.J.; Santulli, R.B.; Smith, C.A.; et al. Neuroanatomic substrates of semantic memory impairment in Alzheimers disease: Patterns of functional MRI activation. J. Int. Neuropsychol. Soc. 1999, 5, 377–392. [Google Scholar] [CrossRef] [PubMed]






| Sex | People | Mean and Standard | Mean and Standard | |
|---|---|---|---|---|
| Deviation of Age | Deviation of Moca | |||
| HC | 68.33 + 5.47 | 28.533 + 1.45 | ||
| Male | 5 | |||
| Female | 10 | |||
| AD | 79.92 + 4.39 | 13.64 + 6.78 | ||
| Male | 4 | |||
| Female | 10 |
| Region Label | Extent | T Value | MNI Coordinate | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R fusiform gyrus | 11,475 | 12.7884 | 42 | −60 | −14 |
| L fusiform gyrus | 11,475 | 10.5606 | −20 | −90 | −2 |
| L cerebellum (III) | 106 | 5.8934 | −4 | −48 | −14 |
| L IFG (p. triangularis) | 1018 | 5.7927 | −38 | 32 | 10 |
| L precentral gyrus | 1018 | 4.955 | −46 | 0 | 48 |
| L posterior-medial frontal | 264 | 5.7539 | 0 | 8 | 62 |
| L IFG (p. orbitalis) | 210 | 5.591 | −52 | 20 | −2 |
| R insula lobe | 316 | 5.479 | 36 | 20 | 4 |
| R temporal pole | 316 | 4.8344 | 58 | 10 | 2 |
| R precentral gyrus | 278 | 5.2847 | 52 | 8 | 44 |
| Region Label | Extent | T Value | MNI Coordinate | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L fusiform gyrus | 5421 | 7.300 | −32 | −76 | −12 |
| R lingual gyrus | 5421 | 7.288 | 22 | −84 | −6 |
| R fusiform gyrus | 5421 | 7.237 | 36 | −56 | −12 |
| L putamen | 24 | 5.508 | −26 | −22 | 4 |
| L precentral gyrus | 114 | 5.110 | −52 | 2 | 46 |
| R middle frontal gyrus | 13 | 4.520 | 42 | 6 | 46 |
| R thalamus | 9 | 4.497 | 28 | −28 | 6 |
| L superior parietal lobule | 24 | 4.480 | −22 | −68 | 50 |
| L middle occipital gyrus | 40 | 4.468 | −24 | −60 | 42 |
| L IFG (p. triangularis) | 6 | 4.284 | −52 | ||
| Region Label | Extent | T Value | MNI Coordinate | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R lingual gyrus | 138 | 5.034 | 24 | V82 | 6 |
| R cerebellum (crus 1) | 70 | 4.476 | 42 | V64 | V24 |
| L inferior occipital gyrus | 36 | 4.311 | V24 | V92 | 0 |
| R cerebellum (VI) | 42 | 4.192 | 24 | V78 | V18 |
| L cerebellum (VI) | 37 | 4.028 | V36 | V56 | V32 |
| L caudate | 10 | 3.971 | V14 | V28 | 26 |
| L cerebellum (VI) | 6 | 3.797 | V8 | V62 | V18 |
| R lingual gyrus | 9 | 3.716 | 14 | V86 | V8 |
| L middle occipital gyrus | 40 | 4.468 | V24 | V60 | 42 |
| L IFG (p. triangularis) | 6 | 4.284 | V52 | 24 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Hou, C.-J.; Derek, N.; Huang, M.-W. fMRI Investigation of Semantic Lexical Processing in Healthy Control and Alzheimer’s Disease Subjects Using Naming Task: A Preliminary Study. Brain Sci. 2021, 11, 718. https://doi.org/10.3390/brainsci11060718
Chen Y-T, Hou C-J, Derek N, Huang M-W. fMRI Investigation of Semantic Lexical Processing in Healthy Control and Alzheimer’s Disease Subjects Using Naming Task: A Preliminary Study. Brain Sciences. 2021; 11(6):718. https://doi.org/10.3390/brainsci11060718
Chicago/Turabian StyleChen, Yen-Ting, Chun-Ju Hou, Natan Derek, and Min-Wei Huang. 2021. "fMRI Investigation of Semantic Lexical Processing in Healthy Control and Alzheimer’s Disease Subjects Using Naming Task: A Preliminary Study" Brain Sciences 11, no. 6: 718. https://doi.org/10.3390/brainsci11060718