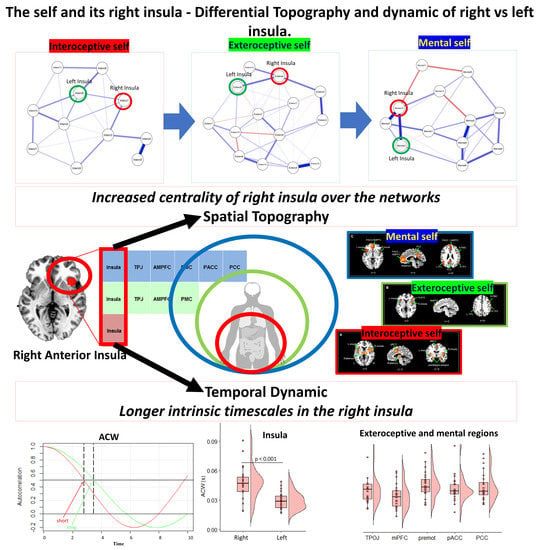
The Self and Its Right Insula—Differential Topography and Dynamic of Right vs. Left Insula
Abstract
:1. Introduction
2. Materials and Methods
2.1. fMRI
2.1.1. fMRI Sample
2.1.2. Resting State fMRI
2.1.3. Task fMRI
2.1.4. fMRI Data Acquisition and Preprocessing
2.1.5. Definition of Regions/Nodes of Interest (ROIs)
2.1.6. Resting State Analysis
2.1.7. Task Functional Connectivity—gPPI
2.2. EEG
2.2.1. EEG Sample
2.2.2. Resting State
2.2.3. Stimuli
2.2.4. Morphing Task
2.2.5. EEG Data Acquisition and Preprocessing
2.2.6. ROIs and Source Localization eLORETA
2.2.7. Autocorrelation Window
2.2.8. Statistical Analysis
3. Results
3.1. Resting State fMRI Analysis
3.2. Task Context-Dependent Functional Connectivity
3.3. Temporal Analyses in EEG
4. Discussion
4.1. From Functional Connectivity over Functional Integration to Spatial Nestedness of Self
4.2. From Autocorrelation Window over Temporal Integration to Temporal Continuity of Self
4.3. Methodological Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, J.; Humphreys, G. The Integrative Self: How Self-Reference Integrates Perception and Memory. Trends Cogn. Sci. 2015, 19, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Northoff, G. Is the Self a Higher-Order or Fundamental Function of the Brain? The “Basis Model of Self-Specificity” and Its Encoding by the Brain’s Spontaneous Activity. Cogn. Neurosci. 2016, 7, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Bermpohl, F. Cortical Midline Structures and the Self. Trends Cogn. Sci. 2004, 8, 102–107. [Google Scholar] [CrossRef]
- Frewen, P.; Schroeter, M.L.; Riva, G.; Cipresso, P.; Fairfield, B.; Padulo, C.; Kemp, A.H.; Palaniyappan, L.; Owolabi, M.; Kusi-Mensah, K.; et al. Neuroimaging the Consciousness of Self: Review, and Conceptual-Methodological Framework. Neurosci. Biobehav. Rev. 2020, 112, 164–212. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the Self in the Brain’s Default Mode Network. NeuroImage 2016, 132, 390–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, R.J.; Schaer, M.; Debbané, M. Degrees of Separation: A Quantitative Neuroimaging Meta-Analysis Investigating Self-Specificity and Shared Neural Activation between Self- and Other-Reflection. Neurosci. Biobehav. Rev. 2012, 36, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Modinos, G.; Ormel, J.; Aleman, A. Activation of Anterior Insula during Self-Reflection. PLoS ONE 2009, 4, e4618. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Ebisch, S.J.H.; Huang, Z.; Di Plinio, S.; Perrucci, M.G.; Romani, G.L.; Mucci, C.; Northoff, G. Spontaneous Brain Activity Predicts Task-Evoked Activity During Animate Versus Inanimate Touch. Cereb. Cortex 2019, 29, 4628–4645. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Huang, Z.; Mucci, C.; Perrucci, M.G.; Ferretti, A.; Fossati, A.; Romani, G.L.; Northoff, G.; Ebisch, S.J.H. How Spontaneous Brain Activity and Narcissistic Features Shape Social Interaction. Sci. Rep. 2017, 7, 9986. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Northoff, G. How Is Our Self Related to Midline Regions and the Default-Mode Network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Enzi, B.; de Greck, M.; Prösch, U.; Tempelmann, C.; Northoff, G. Is Our Self Nothing but Reward? Neuronal Overlap and Distinction between Reward and Personal Relevance and Its Relation to Human Personality. PLoS ONE 2009, 4, e8429. [Google Scholar] [CrossRef]
- Babo-Rebelo, M.; Wolpert, N.; Adam, C.; Hasboun, D.; Tallon-Baudry, C. Is the Cardiac Monitoring Function Related to the Self in Both the Default Network and Right Anterior Insula? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160004. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Duncan, N.W.; de Greck, M.; Northoff, G. Is There a Core Neural Network in Empathy? An FMRI Based Quantitative Meta-Analysis. Neurosci. Biobehav. Rev. 2011, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- D’Argembeau, A.; Collette, F.; Van der Linden, M.; Laureys, S.; Del Fiore, G.; Degueldre, C.; Luxen, A.; Salmon, E. Self-Referential Reflective Activity and Its Relationship with Rest: A PET Study. NeuroImage 2005, 25, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.D. Interoception: The Sense of the Physiological Condition of the Body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Craig, A.D. (Bud) The Sentient Self. Brain Struct. Funct. 2010, 214, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, M.; Northoff, G. Linking Bodily, Environmental and Mental States in the Self—A Three-Level Model Based on a Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 115, 77–95. [Google Scholar] [CrossRef]
- Blanke, O.; Slater, M.; Serino, A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron 2015, 88, 145–166. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Xu, J.; Northoff, G. What COVID -19 Tells Us about the Self: The Deep Intersubjective and Cultural Layers of Our Brain. Psychiatry Clin. Neurosci. 2021, 75, 37–45. [Google Scholar] [CrossRef]
- Northoff, G.; Wainio-Theberge, S.; Evers, K. Spatiotemporal Neuroscience–What Is It and Why We Need It. Phys. Life Rev. 2020, 33, 78–87. [Google Scholar] [CrossRef]
- Northoff, G.; Wainio-Theberge, S.; Evers, K. Is Temporo-Spatial Dynamics the “Common Currency” of Brain and Mind? In Quest of “Spatiotemporal Neuroscience.”. Phys. Life Rev. 2020, 33, 34–54. [Google Scholar] [CrossRef]
- Golesorkhi, M.; Gomez-Pilar, J.; Tumati, S.; Fraser, M.; Northoff, G. Temporal Hierarchy of Intrinsic Neural Timescales Converges with Spatial Core-Periphery Organization. Commun. Biol. 2021, 4, 277. [Google Scholar] [CrossRef]
- Golesorkhi, M.; Gomez-Pilar, J.; Zilio, F.; Berberian, N.; Wolff, A.; Yagoub, M.C.E.; Northoff, G. The Brain and Its Time: Intrinsic Neural Timescales Are Key for Input Processing. Commun. Biol. 2021, 4, 970. [Google Scholar] [CrossRef] [PubMed]
- Zilio, F.; Gomez-Pilar, J.; Cao, S.; Zhang, J.; Zang, D.; Qi, Z.; Tan, J.; Hiromi, T.; Wu, X.; Fogel, S.; et al. Are Intrinsic Neural Timescales Related to Sensory Processing? Evidence from Abnormal Behavioral States. NeuroImage 2021, 226, 117579. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.; Walsh, K.S.O.; Greischar, L.L.; Alexander, A.L.; Fox, A.S.; Davidson, R.J.; Oakes, T.R. Motion Correction and the Use of Motion Covariates in Multiple-subject FMRI Analysis. Hum. Brain. Mapp. 2006, 27, 779–788. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Chai, X.J.; Castañón, A.N.; Öngür, D.; Whitfield-Gabrieli, S. Anticorrelations in Resting State Networks without Global Signal Regression. NeuroImage 2012, 59, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijk, K.R.A.; Hedden, T.; Venkataraman, A.; Evans, K.C.; Lazar, S.W.; Buckner, R.L. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. J. Neurophsyiology 2010, 103, 297–321. [Google Scholar] [CrossRef] [PubMed]
- He, B.J. Scale-Free Properties of the Functional Magnetic Resonance Imaging Signal during Rest and Task. J. Neurosci. 2011, 31, 13786–13795. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Fox, M.D. Towards a Consensus Regarding Global Signal Regression for Resting State Functional Connectivity MRI. NeuroImage 2017, 154, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Vai, B.; Poletti, S.; Damiani, S.; Mucci, C.; Colombo, C.; Zanardi, R.; Benedetti, F.; Northoff, G. All Roads Lead to the Default-Mode Network—Global Source of DMN Abnormalities in Major Depressive Disorder. Neuropsychopharmacology 2020, 45, 2058–2069. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Sparse Inverse Covariance Estimation with the Graphical Lasso. Biostatistics 2008, 9, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating Psychological Networks and Their Accuracy: A Tutorial Paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. Qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef] [Green Version]
- McLaren, D.G.; Ries, M.L.; Xu, G.; Johnson, S.C. A Generalized Form of Context-Dependent Psychophysiological Interactions (GPPI): A Comparison to Standard Approaches. NeuroImage 2012, 61, 1277–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, J.P.; Freund, S.; Hamilton, R.H.; Ganis, G.; Pascual-Leone, A. Hand Response Differences in a Self-Face Identification Task. Neuropsychologia 2000, 38, 1047–1053. [Google Scholar] [CrossRef]
- Heuer, K.; Lange, W.-G.; Isaac, L.; Rinck, M.; Becker, E.S. Morphed Emotional Faces: Emotion Detection and Misinterpretation in Social Anxiety. J. Behav. Ther. Exp. Psychiatry 2010, 41, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Hanoteau, C.; Depy, D.; Rossion, B.; Bruyer, R.; Crommelinck, M.; Guerit, J.M. Right N170 Modulation in a Face Discrimination Task: An Account for Categorical Perception of Familiar Faces. Psychophysiology 2000, 37, 796–806. [Google Scholar] [CrossRef]
- Sandsten, K.E.; Nordgaard, J.; Kjaer, T.W.; Gallese, V.; Ardizzi, M.; Ferroni, F.; Petersen, J.; Parnas, J. Altered Self-Recognition in Patients with Schizophrenia. Schizophr. Res. 2020, 218, 116–123. [Google Scholar] [CrossRef]
- Miller, J.G.; Shrestha, S.; Reiss, A.L.; Vrtička, P. Neural Bases of Social Feedback Processing and Self–Other Distinction in Late Childhood: The Role of Attachment and Age. Cogn. Affect. Behav. Neurosci. 2020, 20, 503–520. [Google Scholar] [CrossRef]
- Wilber GIMP-GIMP 2.10.12 Released. Available online: https://www.gimp.org/news/2019/06/12/gimp-2-10-12-released/ (accessed on 26 August 2021).
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Marcus, D.J.; Westerlund, A.; Casey, B.; Nelson, C. The NimStim Set of Facial Expressions: Judgments from Untrained Research Participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Abrosoft FantaMorph-Photo Morphing Software for Creating Morphing Photos and Animations. Available online: https://www.fantamorph.com/ (accessed on 21 November 2020).
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Winkler, I.; Haufe, S.; Tangermann, M. Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behav. Brain Funct. 2011, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low Resolution Electromagnetic Tomography: A New Method for Localizing Electrical Activity in the Brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Bernacchia, A.; Freedman, D.; Romo, R.; Wallis, J.; Cai, X.; Padoa Schioppa, C.; Pasternak, T.; Seo, H.; Lee, D.; et al. A Hierarchy of Intrinsic Timescales across Primate Cortex. Nat. Neurosci. 2014, 17, 1661–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honey, C.J.; Thesen, T.; Donner, T.H.; Silbert, L.J.; Carlson, C.E.; Devinsky, O.; Doyle, W.K.; Rubin, N.; Heeger, D.J.; Hasson, U. Slow Cortical Dynamics and the Accumulation of Information over Long Timescales. Neuron 2012, 76, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, R.J.; Debbané, M.; Fox, P.T.; Bzdok, D.; Eickhoff, S.B. Functional Connectivity Mapping of Regions Associated with Self- and Other-Processing. Hum. Brain Mapp. 2015, 36, 1304–1324. [Google Scholar] [CrossRef] [Green Version]
- Deco, G.; Tononi, G.; Boly, M.; Kringelbach, M.L. Rethinking Segregation and Integration: Contributions of Whole-Brain Modelling. Nat. Rev. Neurosci. 2015, 16, 430–439. [Google Scholar] [CrossRef]
- Scalabrini, A.; Mucci, C.; Esposito, R.; Damiani, S.; Northoff, G. Dissociation as a Disorder of Integration–On the Footsteps of Pierre Janet. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 101, 109928. [Google Scholar] [CrossRef]
- Wolff, A.; Di Giovanni, D.A.; Gómez-Pilar, J.; Nakao, T.; Huang, Z.; Longtin, A.; Northoff, G. The Temporal Signature of Self: Temporal Measures of Resting-State EEG Predict Self-Consciousness. Hum. Brain Mapp. 2019, 40, 789–803. [Google Scholar] [CrossRef] [Green Version]
- Kolvoort, I.R.; Wainio-Theberge, S.; Wolff, A.; Northoff, G. Temporal Integration as “Common Currency” of Brain and Self-Scale-Free Activity in Resting-State EEG Correlates with Temporal Delay Effects on Self-Relatedness. Hum. Brain Mapp. 2020, 41, 4355–4374. [Google Scholar] [CrossRef] [PubMed]
- Himberger, K.D.; Chien, H.-Y.; Honey, C.J. Principles of Temporal Processing Across the Cortical Hierarchy. Neuroscience 2018, 389, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Ersner-Hershfield, H.; Wimmer, G.E.; Knutson, B. Saving for the Future Self: Neural Measures of Future Self-Continuity Predict Temporal Discounting. Soc. Cogn. Affect. Neurosci. 2009, 4, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northoff, G.; Magioncalda, P.; Martino, M.; Lee, H.-C.; Tseng, Y.-C.; Lane, T. Too Fast or Too Slow? Time and Neuronal Variability in Bipolar Disorder—A Combined Theoretical and Empirical Investigation. Schizophr. Bull. 2018, 44, 54–64. [Google Scholar] [CrossRef] [Green Version]
- (Bud) Craig, A.D. Significance of the Insula for the Evolution of Human Awareness of Feelings from the Body. Ann. N. Y. Acad. Sci. 2011, 1225, 72–82. [Google Scholar] [CrossRef]
- (Bud) Craig, A.D. How Do You Feel—Now? The Anterior Insula and Human Awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Tumati, S.; Northoff, G. Rest-Task Modulation of FMRI-Derived Global Signal Topography Is Mediated by Transient Coactivation Patterns. PLoS Biol. 2020, 18, e3000733. [Google Scholar] [CrossRef] [PubMed]
- Wiebking, C.; Northoff, G. Interoceptive Awareness and the Insula–Application of Neuroimaging Techniques in Psychotherapy. GSTF J. Psychol. JPsych 2014, 1. [Google Scholar]
- Wiebking, C.; Duncan, N.W.; Tiret, B.; Hayes, D.J.; Marjaǹska, M.; Doyon, J.; Bajbouj, M.; Northoff, G. GABA in the Insula—A Predictor of the Neural Response to Interoceptive Awareness. NeuroImage 2014, 86, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Wiebking, C.; de Greck, M.; Duncan, N.W.; Tempelmann, C.; Bajbouj, M.; Northoff, G. Interoception in Insula Subregions as a Possible State Marker for Depression—an Exploratory FMRI Study Investigating Healthy, Depressed and Remitted Participants. Front. Behav. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalabrini, A.; Wolman, A.; Northoff, G. The Self and Its Right Insula—Differential Topography and Dynamic of Right vs. Left Insula. Brain Sci. 2021, 11, 1312. https://doi.org/10.3390/brainsci11101312
Scalabrini A, Wolman A, Northoff G. The Self and Its Right Insula—Differential Topography and Dynamic of Right vs. Left Insula. Brain Sciences. 2021; 11(10):1312. https://doi.org/10.3390/brainsci11101312
Chicago/Turabian StyleScalabrini, Andrea, Angelika Wolman, and Georg Northoff. 2021. "The Self and Its Right Insula—Differential Topography and Dynamic of Right vs. Left Insula" Brain Sciences 11, no. 10: 1312. https://doi.org/10.3390/brainsci11101312