Characteristics of Clinical Symptoms, Cerebral Images and Stroke Etiology in Vertebro-Basilar Artery Fenestration-Related Infarction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
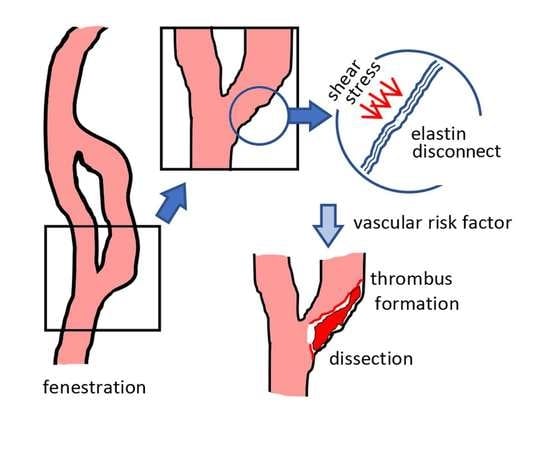
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayrak, A.H.; Senturk, S.; Akay, H.O.; Ozmen, C.A.; Bukte, Y.; Nazaroglu, H. The frequency of intracranial arterial fenestrations: A study with 64-detector CT-angiography. Eur. J. Radiol. 2011, 77, 392–396. [Google Scholar] [CrossRef]
- Teal, J.S.; Rumbaugh, C.L.; Bergeron, R.T.; Segall, H.D. Angiographic demonstration of fenestrations of the intradural intracranial arteries. Radiology 1973, 106, 123–126. [Google Scholar] [CrossRef]
- Wollschlaeger, G.; Wollschlaeger, P.B.; Lucas, F.V.; Lopez, V.F. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1967, 101, 68–87. [Google Scholar] [CrossRef]
- Bharatha, A.; Aviv, R.I.; White, J.; Fox, A.J.; Symons, S.P. Intracranial arterial fenestrations: Frequency on CT angiography and association with other vascular lesions. Surg. Radiol. Anat. 2008, 30, 397–401. [Google Scholar] [CrossRef]
- Cademartiri, F.; Stojanov, D.; Dippel, D.W.; Van Der Lugt, A.; Tanghe, H. Noninvasive detection of a ruptured aneurysm at a basilar artery fenestration with submillimeter multisection CT angiography. AJNR Am. J. Neuroradiol. 2003, 24, 2009–2010. [Google Scholar]
- Gao, L.Y.; Guo, X.; Zhou, J.J.; Zhang, Q.; Fu, J.; Chen, W.J.; Yang, Y.J. Basilar artery fenestration detected with CT angiography. Eur. Radiol. 2013, 23, 2861–2867. [Google Scholar] [CrossRef]
- Tanaka, M.; Kikuchi, Y.; Ouchi, T. Neuroradiological analysis of 23 cases of basilar artery fenestration based on 2280 cases of MR angiographies. Interv. Neuroradiol. 2006, 12, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, L.; He, X.; Zhang, X.; Hu, L.; Du, B.; Wang, W.; Jiang, W.; Liu, Z. Wall thickening pattern in atherosclerotic basilar artery stenosis. Neurol. Sci. 2016, 37, 269–276. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.B.; Lu, S.; Liu, Z.J.; Zhu, X.J. Plaque distribution of basilar artery fenestration by 3D High-resolution MR vessel wall imaging. Cell Transplant. 2019, 28, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.; Fox, A.J.; Vinuela, F.; Lylyk, P.; Ferguson, G.G.; Drake, C.G.; Peerless, S.J. Saccular aneurysms in basilar artery fenestration. AJNR Am. J. Neuroradiol. 1987, 8, 233–236. [Google Scholar]
- Peluso, J.P.; van Rooij, W.J.; Sluzewski, M.; Beute, G.N. Aneurysms of the vertebrobasilar junction: Incidence, clinical presentation, and outcome of endovascular treatment. AJNR Am. J. Neuroradiol. 2007, 28, 1747–1751. [Google Scholar] [CrossRef] [Green Version]
- Rinkel, G.J. Natural history, epidemiology and screening of unruptured intracranial aneurysms. J. Neuroradiol. 2008, 35, 99–103. [Google Scholar] [CrossRef]
- Bozek, P.; Pilch-Kowalczyk, J.; Kluczewska, E.; Zymon-Zagorska, A. Detection of cerebral artery fenestrations by computed tomography angiography. Neurol. Neurochir. Pol. 2012, 46, 239–244. [Google Scholar] [CrossRef]
- Kachhara, R.; Nair, S.; Gupta, A.K. Fenestration of the proximal anterior cerebral artery (A1) with aneurysm manifesting as subarachnoid hemorrhage—Case report. Neurol. Med. Chir. (Tokyo) 1998, 38, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Kloska, S.P.; Schlegel, P.M.; Strater, R.; Niederstadt, T.U. Causality of pediatric brainstem infarction and basilar artery fenestration? Pediatr. Neurol. 2006, 35, 436–438. [Google Scholar] [CrossRef]
- Gold, J.J.; Crawford, J.R. An unusual cause of pediatric stroke secondary to congenital basilar artery fenestration. Case Rep. Crit. Care 2013, 2013, 627972. [Google Scholar] [CrossRef]
- Palazzo, P.; Ruff, M.; Lyerly, M.J.; Alexandrov, A.V. Basilar artery thrombus vs. fenestration: A differential diagnostic challenge in acute ischemic stroke. J. Neuroimaging 2014, 24, 607–609. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar]
- Sato, S.; Toyoda, K.; Matsuoka, H.; Okatsu, H.; Kasuya, J.; Takada, T.; Shimode, A.; Uehara, T.; Naritomi, H.; Minematsu, K. Isolated anterior cerebral artery territory infarction: Dissection as an etiological mechanism. Cerebrovasc. Dis. 2010, 29, 170–177. [Google Scholar] [CrossRef]
- Bernard, T.J.; Mull, B.R.; Handler, M.H.; Harned, R.K.; Filley, C.M.; Kumpe, D.A.; Tseng, B.S. An 18-year-old man with fenestrated vertebral arteries, recurrent stroke and successful angiographic coiling. J. Neurol. Sci. 2007, 260, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Meinel, T.R.; Pult, F.; Gralla, J.; Arnold, M.; Bassetti, C.; Jung, S. Successful endovascular recanalization of a partially occluded basilar artery fenestration. Interv. Neuroradiol. 2019, 25, 44–46. [Google Scholar] [CrossRef]
- Wu, X.; Lin, A.; Zhu, J.; Cai, B. Basilar artery fenestration: An unusual possible cause of ischaemic stroke? BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Horie, N.; Tsunoda, K.; Tateishi, Y.; Izumo, T.; Hayashi, K.; Tsujino, A.; Nagata, I. Bow hunter’s stroke due to stretching of the vertebral artery fenestration: A case report. NMC Case Rep. J. 2015, 2, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, R.; Koyama, K.; Kurokawa, T.; Kurosawa, Y. Brainstem infarction due to vertebral artery fenestration in a young adult man: A case report. Jpn. J. Stroke 2010, 32, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Debette, S.; Compter, A.; Labeyrie, M.A.; Uyttenboogaart, M.; Metso, T.M.; Majersik, J.J.; Goeggel-Simonetti, B.; Engelter, S.T.; Pezzini, A.; Bijlenga, P.; et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015, 14, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Finlay, H.M.; Canham, P.B. The layered fabric of cerebral artery fenestrations. Stroke 1994, 25, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Nakatomi, H.; Tsutsumi, K.; Inoue, T.; Teraoka, A.; Yoshimoto, Y.; Ide, T.; Kitanaka, C.; Ueki, K.; Imai, H.; et al. Symptomatic recurrence of intracranial arterial dissections: Follow-up study of 143 consecutive cases and pathological investigation. Stroke 2013, 44, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Ro, A.; Kageyama, N.; Abe, N.; Takatsu, A.; Fukunaga, T. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: Clinical and histopathological investigations from a medicolegal perspective. J. Neurosurg. 2009, 110, 948–954. [Google Scholar] [CrossRef]
- Mizutani, T.; Kojima, H.; Asamoto, S.; Miki, Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J. Neurosurg. 2001, 94, 712–717. [Google Scholar] [CrossRef]
- Tsuei, Y.S.; Matsumoto, Y.; Ohta, M.; Nakayama, T.; Ezura, M.; Takahashi, A. Vertebrobasilar junction fenestration with dumbbell-shaped aneurysms formation: Computational fluid dynamics analysis. Surg. Neurol. 2009, 72 (Suppl. 2), S11–S19. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsumoto, S. [Fenestration of the internal carotid artery (author’s transl)]. Neurol. Med. Chir. (Tokyo) 1982, 22, 291–294. [Google Scholar] [CrossRef]
- Sun, Z.K.; Li, M.; Li, M.H.; Li, Y.D.; Sun, W.P.; Zhu, Y.Q. Fenestrations accompanied by intracranial aneurysms assessed with magnetic resonance angiography. Neurol. India 2012, 60, 45–49. [Google Scholar] [CrossRef]
- Giannoglou, G.D.; Soulis, J.V.; Farmakis, T.M.; Farmakis, D.M.; Louridas, G.E. Haemodynamic factors and the important role of local low static pressure in coronary wall thickening. Int. J. Cardiol. 2002, 86, 27–40. [Google Scholar] [CrossRef]
- Berry, A.D., 3rd; Kepes, J.J.; Wetzel, M.D. Segmental duplication of the basilar artery with thrombosis. Stroke 1988, 19, 256–260. [Google Scholar] [CrossRef] [Green Version]

| Author (et al.) | Age | Sex | Stroke Etiology | Fenestration Classification | Headache | Infarct Area | Findings Suggesting Dissection on MRA | Intramural Hematoma on T1WI | D-Dimer (μg/mL) | Hypertension | Dyslipidemia | Diabetes | Atrial Filiation | Related Injury | Worsening | Vertigo/Dizziness | Ataxia | Weakness | Dysesthesia | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| String | Pearl | Double Lumen | Shape Change | |||||||||||||||||||
| Case 1 | 71 | F | D | I | + | pons, cerebellar | + | - | - | + | - | 2.1 | + | + | - | - | - | - | + | + | - | - |
| Case 2 | 74 | M | D | III | + | Medulla PICA | + | - | - | + | + | <1.0 | + | - | - | - | - | - | + | + | - | - |
| Case 3 | 61 | M | D | III | - | cerebellar | + | - | - | + | - | 1.1 | + | - | - | - | - | - | + | - | - | - |
| Case 4 | 71 | M | D | I | + | medulla | + | - | - | + | + | 1.5 | + | + | - | + | - | - | + | - | + | + |
| Bernard [20] | 18 | M | D | (VA) | + | cerebellar | + | + | - | + | NA | NA | - | - | - | - | - | + | + | - | - | - |
| Gold [16] | 12 | M | E | II | - | cerebellar | - | - | - | NA | NA | NA | - | - | - | - | - | - | + | + | - | - |
| Kloska [15] | 5 | M | E | I | + | pons | + | - | - | + | NA | NA | - | - | - | - | - | - | - | - | + | - |
| Meinnel [21] | 76 | M | E | IV | - | pons | + | - | - | + | NA | NA | + | + | - | - | - | - | - | - | + | + |
| Palazzo [17] | 56 | M | E | I | - | cerebellar posterior lobe | + | - | - | + | NA | NA | + | - | - | - | - | - | + | + | - | - |
| Wu [22] | 36 | M | E | IV | - | Cerebellar (vermis) | - | - | - | + | NA | NA | - | - | - | - | - | - | + | + | - | - |
| Yamaguchi [23] | 45 | M | C | (VA) | - | Pons cerebellar | + | - | - | + | NA | NA | - | - | - | - | - | - | + | - | - | - |
| Yamamoto [24] | 30 | M | E | (VA) | + | midbrain | + | - | - | + | NA | <1.0 | - | - | - | - | - | - | + | - | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, N.; Ueno, Y.; Hira, K.; Kijima, C.; Nakajima, S.; Yamashiro, K.; Hattori, N. Characteristics of Clinical Symptoms, Cerebral Images and Stroke Etiology in Vertebro-Basilar Artery Fenestration-Related Infarction. Brain Sci. 2020, 10, 243. https://doi.org/10.3390/brainsci10040243
Miyamoto N, Ueno Y, Hira K, Kijima C, Nakajima S, Yamashiro K, Hattori N. Characteristics of Clinical Symptoms, Cerebral Images and Stroke Etiology in Vertebro-Basilar Artery Fenestration-Related Infarction. Brain Sciences. 2020; 10(4):243. https://doi.org/10.3390/brainsci10040243
Chicago/Turabian StyleMiyamoto, Nobukazu, Yuji Ueno, Kenichiro Hira, Chikage Kijima, Sho Nakajima, Kazuo Yamashiro, and Nobutaka Hattori. 2020. "Characteristics of Clinical Symptoms, Cerebral Images and Stroke Etiology in Vertebro-Basilar Artery Fenestration-Related Infarction" Brain Sciences 10, no. 4: 243. https://doi.org/10.3390/brainsci10040243