Engineering Biomimetic Gelatin Based Nanostructures as Synthetic Substrates for Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
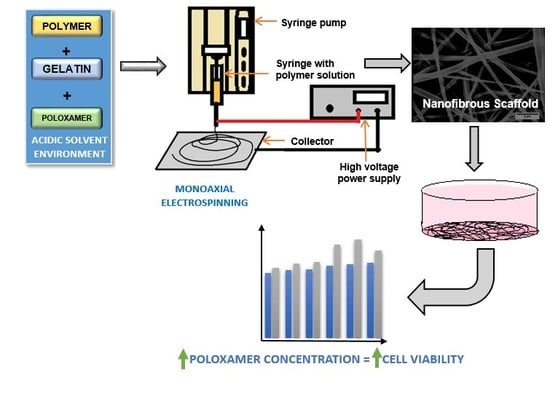
2.2. Preparation of Solutions
2.3. Scanning Electron Microscopy Studies
2.4. Atomic Force Microscopy Studies
2.5. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) Studies
2.6. Cell Viability Studies
2.7. Statistical Analysis
3. Results
3.1. Morphology of PCL/Gelatin/Poloxamer 188 (P188) Scaffolds in Comparison with PCL/Gelatin and PCL Nanofibers
3.2. Chemical Characteristics of the Scaffolds to Determine the Presence of P188 and Gelatin in the Composites
3.3. Cell Viability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merker, H. Morphology of the basement membrane. Microsc. Res. Tech. 1994, 28, 95–124. [Google Scholar] [CrossRef]
- Morrissey, M.; Sherwood, D. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler, A.; Vanacore, R.; Chetyrkin, S.; Pedchenko, V.; Bhave, G.; Yin, V.; Stothers, C.; Rose, K.; McDonald, W.; Clark, T.; et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA 2013, 111, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, T.; Gonda, T. Distribution of the Pores of Epithelial Basement Membrane in the Rat Small Intestine. J. Vet. Med Sci. 2004, 66, 695–700. [Google Scholar] [CrossRef]
- Mestres, P.; Gomez, L.; Lopez, T.; del Rosario, G.; Lukas, S.; Hartmann, U. The basement membrane of the isolated rat colonic mucosa. A light, electron and atomic force microscopy study. Ann. Anat. 2014, 196, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Cole, G.; Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010, 29, 402–410. [Google Scholar] [CrossRef]
- Van Agtmael, T.; Bruckner-Tuderman, L. Basement membranes and human disease. Cell Tissue Res. 2009, 339, 167–188. [Google Scholar] [CrossRef] [Green Version]
- Kayaselcuk, F.; Serin, E.; Gumurdulu, Y.; Ozer, B.; Tuncer, I.; Boyacioglu, S. Subepithelial basement membrane thickness in patients with normal colonic mucosal appearance in colonoscopy: Results from southern Turkey. World J. Gastroenterol. 2004, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Eltboli, O.; Mistry, V.; Barker, B.; Brightling, C. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology 2015, 20, 667–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vllasaliu, D.; Fowler, R.; Garnett, M.; Eaton, M.; Stolnik, S. Barrier characteristics of epithelial cultures modelling the airway and intestinal mucosa: A comparison. Biochem. Biophys. Res. Commun. 2011, 415, 579–585. [Google Scholar] [CrossRef]
- Moradi, E.; Vllasaliu, D.; Garnett, M.; Falcone, F.; Stolnik, S. Ligand density and clustering effects on endocytosis of folate modified nanoparticles. RSC Adv. 2012, 2, 3025. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Falcone, F.; Stolnik, S.; Garnett, M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp. Cell Res. 2014, 323, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Vllasaliu, D.; Thanou, M.; Stolnik, S.; Fowler, R. Recent advances in oral delivery of biologics: Nanomedicine and physical modes of delivery. Expert Opin. Drug Deliv. 2018, 15, 759–770. [Google Scholar] [CrossRef]
- Alfano, M.; Chasens, A.; Masi, C. Autoradiographic study of the penetration of radiolabelled dextrans and inulin through non-keratinized oral mucosain vitro. J. Periodontal Res. 1977, 12, 368–377. [Google Scholar] [CrossRef]
- Fowler, R.; Vllasaliu, D.; Trillo, F.; Garnett, M.; Alexander, C.; Horsley, H.; Smith, B.; Whitcombe, I.; Eaton, M.; Stolnik, S. Nanoparticle Transport in Epithelial Cells: Pathway Switching Through Bioconjugation. Small 2013, 9, 3282–3294. [Google Scholar] [CrossRef]
- Dix, C.; Hassan, I.; Obray, H.; Shah, R.; Wilson, G. The Transport of Vitamin B12 Through Polarized Monolayers of Caco-2 Cells. Gastroenterology 1990, 98, 1272–1279. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Ma, T.; Boivin, M.; Ye, D.; Pedram, A.; Said, H. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef]
- Denning, D.; Roos, W. Elucidating the molecular mechanisms underlying cellular response to biophysical cues using synthetic biology approaches. Cell Adhes. Migr. 2016, 10, 540–553. [Google Scholar] [CrossRef] [Green Version]
- Flemming, R.; Murphy, C.; Abrams, G.; Goodman, S.; Nealey, P. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; García, A. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef]
- Kajdič, S.; Vrečer, F.; Kocbek, P. Preparation of poloxamer-based nanofibers for enhanced dissolution of carvedilol. Eur. J. Pharm. Sci. 2018, 117, 331–340. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, H.; Lim, C.; Ramakrishna, S.; Huang, Z. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. 2004, 72B, 156–165. [Google Scholar] [CrossRef]
- Chung, T.; Liu, D.; Wang, S.; Wang, S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Engler, A.; Sen, S.; Sweeney, H.; Discher, D. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Goddard, J.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Jung, H.; Kwak, B.; Yang, H.; Tae, G.; Kim, J.; Shin, K. Attachment of cells to poly(styrene-co-acrylic acid) thin films with various charge densities. Colloids Surf. A 2008, 313–314, 562–566. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Kang, I.; Lee, H. Cell behaviour on polymer surfaces with different functional groups. Biomaterials 1994, 15, 705–711. [Google Scholar] [CrossRef]
- Khatiwala, C.; Peyton, S.; Metzke, M.; Putnam, A. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J. Cell. Physiol. 2007, 211, 661–672. [Google Scholar] [CrossRef]
- Khoda, A. Engineered Tissue Scaffolds with Variational Porous Architecture. J. Biomech. Eng. 2010, 133, 011001. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.; Mishra, N. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Yu, J.; Liu, S.; Gu, P. Electrospun chitosan-graft-poly (ε-caprolactone)/poly (ε-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 13–19. [Google Scholar] [CrossRef]
- Li, W.; Cooper, J.; Mauck, R.; Tuan, R. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef]
- Kim, C.; Khil, M.; Kim, H.; Lee, H.; Jahng, K. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part B 2006, 78B, 283–290. [Google Scholar] [CrossRef]
- Barnes, C.; Sell, S.; Boland, E.; Simpson, D.; Bowlin, G. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.; Morshed, M.; Nasr-Esfahani, M.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Kerleta, V. Poloxamer 188 supplemented culture medium increases the vitality of Caco-2 cells after subcultivation and freeze/thaw cycles. ALTEX 2010, 27, 191–197. [Google Scholar] [CrossRef]
- Böttjer, R.; Grothe, T.; Wehlage, D.; Ehrmann, A. Electrospraying poloxamer/(bio-)polymer blends using a needleless electrospinning machine. J. Text. Fibrous Mater. 2018, 1. [Google Scholar] [CrossRef]
- Aytimur, A.; Koçyiğit, S.; Uslu, İ. Synthesis and Characterization of Poly(vinyl alcohol)/Poly(vinyl pyrrolidone)-Iodine Nanofibers with Poloxamer 188 and Chitosan. Polym. Plast. Technol. Eng. 2013, 52, 661–666. [Google Scholar] [CrossRef]
- Denis, P.; Dulnik, J.; Sajkiewicz, P. Electrospinning and Structure of Bicomponent Polycaprolactone/Gelatin Nanofibers Obtained Using Alternative Solvent System. Int. J. Polym. Mater. Polym. Biomater. 2014, 64, 354–364. [Google Scholar] [CrossRef]
- Voytik-Harbin, S.; Brightman, A.; Waisner, B.; Lamar, C.; Badylak, S. Application and evaluation of the alamarblue assay for cell growth and survival of fibroblasts. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 239–246. [Google Scholar] [CrossRef]
- De Oliveira, R.R.L.; Albuquerque, D.A.C.; Leite, F.L.; Yamaji, F.M.; Cruz, T.G.S. Measurement of the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Pham, Q.; Sharma, U.; Mikos, A. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Ekram, B.; Abdel-Hady, B.; El-kady, A.; Amr, S.; Waley, A.; Guirguis, O. Optimum parameters for the production of nano-scale electrospun polycaprolactone to be used as a biomedical material. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045018. [Google Scholar] [CrossRef] [Green Version]
- Yördem, O.; Papila, M.; Menceloğlu, Y. Effects of electrospinning parameters on polyacrylonitrile nanofiber diameter: An investigation by response surface methodology. Mater. Des. 2008, 29, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Hayati, I.; Bailey, A.; Tadros, T. Investigations into the mechanisms of electrohydrodynamic spraying of liquids. J. Colloid Interface Sci. 1987, 117, 205–221. [Google Scholar] [CrossRef]
- Deitzel, J.; Kleinmeyer, J.; Harris, D.; Beck Tan, N. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Cheng, Z.; Teoh, S. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.; Sun, T.; Sultana, N. In VitroBiological Evaluation of Electrospun Polycaprolactone/Gelatine Nanofibrous Scaffold for Tissue Engineering. J. Nanomater. 2015, 2015, 303426. [Google Scholar] [CrossRef]
- Hassan, M.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. 3 Biotech 2017, 7, 249. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Patra, P.; Warner, S.; Bhowmick, S. Role of Fiber Diameter in Adhesion and Proliferation of NIH 3T3 Fibroblast on Electrospun Polycaprolactone Scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef]








| Polymer Solution with 90%/10% Acetic Acid Formic Acid Solvent System | The Concentration of Each Component in the Total Polymer Concentration of 15% (w/v) | ||
|---|---|---|---|
| PCL | Gelatin | Poloxamer 188 | |
| PCL | 100% | - | - |
| PCL/gelatin (90:10) | 90% | 10% | - |
| PCL/gelatin/P188 (85:10:5) | 85% | 10% | 5% |
| PCL/gelatin/P188 (80:10:10) | 80% | 10% | 10% |
| PCL/gelatin/P188 (70:10:20) | 70% | 10% | 20% |
| Average Fiber Diameter (nm) of Fibers Electrospun at | ||||
|---|---|---|---|---|
| Samples | 18 kV | 20 kV | 22 kV | 24 kV |
| PCL | 383 ± 301 | 425 ± 419 | 453 ± 314 | 487 ± 302 |
| PCL/Gelatin (90:10) | 307 ± 109 | 595 ± 340 | 282 ± 190 | 384 ± 245 |
| PCL/ Gelatin/ P188 (85:10:5) | 280 ±126 | 297 ± 181 | 390 ± 158 | 291 ± 109 |
| PCL/ Gelatin/ P188 (80:10:10) | 361 ± 175 | 337 ± 129 | 327 ± 196 | 239 ± 107 |
| PCL/ Gelatin/ P188 (70:10:20) | 363 ± 336 | 463 ± 317 | 306 ± 163 | 227 ± 62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazhanimala, S.K.; Vllasaliu, D.; Raimi-Abraham, B.T. Engineering Biomimetic Gelatin Based Nanostructures as Synthetic Substrates for Cell Culture. Appl. Sci. 2019, 9, 1583. https://doi.org/10.3390/app9081583
Pazhanimala SK, Vllasaliu D, Raimi-Abraham BT. Engineering Biomimetic Gelatin Based Nanostructures as Synthetic Substrates for Cell Culture. Applied Sciences. 2019; 9(8):1583. https://doi.org/10.3390/app9081583
Chicago/Turabian StylePazhanimala, Shaleena K., Driton Vllasaliu, and Bahijja T. Raimi-Abraham. 2019. "Engineering Biomimetic Gelatin Based Nanostructures as Synthetic Substrates for Cell Culture" Applied Sciences 9, no. 8: 1583. https://doi.org/10.3390/app9081583