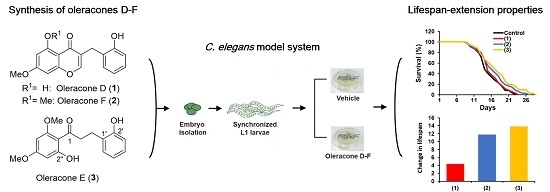
Synthesis and Evaluation of the Lifespan-Extension Properties of Oleracones D–F, Antioxidative Flavonoids from Portulaca oleracea L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
2.1.2. Experimental Section
2.2. Biology
2.2.1. C. elegans Maintenance
2.2.2. Lifespan Assay
2.2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical involvement in aging. Drugs Aging 1993, 3, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: Prospects for further increases in the functional life span. AGE 1994, 17, 119–146. [Google Scholar] [CrossRef]
- Harman, D. Aging and Disease: Extending Functional Life Span. Ann. N. Y. Acad. Sci. 1996, 786, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of againg. J. Gerontol. 1956, 12, 257–263. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxi. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Cherniack, E. The Potential Influence of Plant Polyphenols on the Aging Process. Complementary Med. Res. 2010, 17, 181–187. [Google Scholar] [CrossRef]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.D.C.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.; Henichart, J.; Catteau, J.; Gaydou, E.; Wallet, J. Scavenger and antioxidant properties of ten synthetic flavones. Free Radic. Biol. Med. 1992, 13, 211–219. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Cardoso, S.M.; Ribeiro, M.; Seixas, R.S.; Silva, A.M.; Rego, A.C. Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington’s disease mouse striatal cells. Neurochem. Int. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Alves-Silva, J.M.; Pereira, O.R.; Cardoso, S.M. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Curr. Top. Med. Chem. 2015, 15, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Carvalho, I.S. In vitro antioxidant activity, phenolic compounds and protective effect against DNA damage provided by leaves, stems and flowers of Portulaca oleracea (Purslane). Nat. Prod. Commun. 2014, 9, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Lamoochi, Z.; Moghaddam, H.F.; Mansouri, S.M.T. Effects of Portulaca oleracea ethanolic extract on reproductive system of aging female mice. Int. J. Reprod. Biomed. 2016, 14, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, W.; Ying, X.; Stien, D. New flavonoids from Portulaca oleracea L. And their activities. Fitoterapia 2018, 127, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ying, Z.; Liu, H.; Ying, X.; Yang, G. A new homoisoflavone from Portulaca oleracea L. and its antioxidant activity. Nat. Prod. Res. 2018, 32, 1–7. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Balasubramanian, S.; Nair, M.G. An Efficient “One Pot” Synthesis of Isoflavones. Synth. Commun. 2000, 30, 469–484. [Google Scholar] [CrossRef]
- Kirkiacharian, B.S.; Gomis, M. New Convenient Synthesis of Homoisoflavanones and (±)-Di-O-methyldihydroeucomin. Synth. Commun. 2005, 35, 563–569. [Google Scholar] [CrossRef]
- Kim, Y.S.; Han, Y.T.; Jeon, H.; Cha, D.S. Antiageing properties of Damaurone D in Caenorhabditis elegans. J. Pharm. Pharmacol. 2018, 70, 1423–1429. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.A.; Lim, C.; Cha, D.S.; Han, Y.T. Synthesis and Evaluation of the Lifespan-Extension Properties of Oleracones D–F, Antioxidative Flavonoids from Portulaca oleracea L. Appl. Sci. 2019, 9, 4014. https://doi.org/10.3390/app9194014
Yoon JA, Lim C, Cha DS, Han YT. Synthesis and Evaluation of the Lifespan-Extension Properties of Oleracones D–F, Antioxidative Flavonoids from Portulaca oleracea L. Applied Sciences. 2019; 9(19):4014. https://doi.org/10.3390/app9194014
Chicago/Turabian StyleYoon, Jeong A, Changjin Lim, Dong Seok Cha, and Young Taek Han. 2019. "Synthesis and Evaluation of the Lifespan-Extension Properties of Oleracones D–F, Antioxidative Flavonoids from Portulaca oleracea L." Applied Sciences 9, no. 19: 4014. https://doi.org/10.3390/app9194014