1. Introduction
Tablets of various phosphine (PH
3)-containing chemicals, including aluminum phosphide, release PH
3s, which have been extensively used as fumigants for the control of insect pests, thereby minimizing trading risks and protecting stored agricultural products [
1]. In addition, PH
3s have been introduced as an alternative to methyl bromide for controlling adult greenhouse thrips (
Heliothrips haemorrhoidalis), adult aphids (
Myzus persicae), and light brown apple moth larvae (
Epipyas postvittana), in cut flowers [
2]. Based on the trials, the most effective formulation for aphids was 1000 μL/L PH
3 + 33% CO
2 for 4 h, while all tested insects were killed within 36 h of exposure [
2].
Furthermore,
M. persicae can be controlled by Brassicacaeae ethyl ester oils [
3], a certain wavelength of light-emitting diodes [
4], and biologically using banker plants with
Aphidius colemani [
5]. Recently, ethyl formate (EF) was used to control
M. persicae by fumigation and this fumigant induced 2-fold cytochrome c oxidase (COX) activity in comparison with the control [
6]. Stewart and Mon [
7] introduced the use of EF fumigation to protect film-wrapped lettuces from
M. persicae.
Interestingly, PH
3 and EF have been combined for use in the control of insect pests. The combination of PH
3 and EF effectively killed
Aphis gossypii, the cotton aphid [
8], whereas individual treatments with PH
3 fumigation over short periods of time or at low EF concentrations provided relatively low eradication rates. The combination may also completely control the eggs of
Planococcus citri (Hemiptera: Pseudococcidae) on pineapples, when applied at concentrations of 25.1/1.0 (EF/PH
3) mg/L at 8 °C for 4 h [
9].
This combined fumigation has been used to eradicate
M. persicae efficiently and may hinder the development of PH
3 and/or EF resistance in
M. persicae. PH
3 resistance in various insect pests have been reported throughout the world in various insect species, including
Rhyzopertha dominica,
Tribolium castaneum, and
Cryptolestes ferrugineus [
10,
11,
12], leading farmers and fumigators to explore new fumigation tools to control PH
3-resistant insect pests in storage facilities [
13,
14].
This study assessed the acute toxicity in M. persicae caused by PH3 to determine its lethal concentration LC10 and LC50 values. Regarding the acute toxicity, activities of two enzymes such as acetylcholinesterase (AChE) and cytochrome c oxidase (COX) were measured to know the actual target sites of PH3. AChE and COX have been known to be the target site of an organophosphorus insecticide and PH3, respectively. Besides, expression levels of six genes responsible for expressing cytochrome P450 6CY3 (cyp6cy3), farnesyl diphosphate synthase 1 (fps1), acetylcholinesterase (ache), voltage gated sodium channel subunit 1 (para), nicotinic acetylcholine receptor alpha 3 subunit (ni-acr), and ecdysone receptor (ecr) were also measured to understand effects of PH3 on metabolism, neurotransmission system, and growth in M. persicae. Finally, overall protein expression levels were compared between the PH3-treated adults of M. persicae and the control using liquid-chromatography tandem mass spectrometry (LC-MS/MS) to find further biochemical reasons for the explication of the toxic effect by PH3.
2. Material and Methods
2.1. Chemicals
PH3 was obtained as ECO2Fume™ (2% PH3 + 98% CO2) from Cytec (Sydney, Australia). Acetylthiocholine iodide (ATChI), bovine serum albumin (BSA), cytochrome c, 5,5′-dithiobis (2-nitrobezoic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The DEPC-treated water was purchased from Biosesang (Seongnam, Korea). The Rotor-Gene SYBR Green PCR Kit and QIAzol Lysis reagent were purchased from Qiagen (Düsseldorf, Germany), and the Maxima First Strand cDNA Synthesis Kit with dsDNase was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Insect Strain and Breeding
Adults of Myzus persicae acquired from Quarantine and Inspection Agency (Gimcheon, Korea) were placed on Chinese cabbages, which were grown to a 5 to 7 cm leaf length, for feeding and breeding 17 days after seeding. Chinese cabbages were maintained in a glass greenhouse at 30 ± 1 °C and a relative humidity of 30%. The M. persicae were placed and bred on Chinese cabbages in 5 pots in an acrylic growth cage (30 × 30 × 45 cm) at 25 ± 1 °C and a relative humidity of 50–60%.
2.3. Fumigation Assay of PH3
A fumigation bioassay using a concentration of 0.01 to 1.0 mg/L of PH3 (ECO2Fume™: 2% PH3 + 98% CO2, Cytec, Sydney, Australia) was performed with 30 nymphs or adults of M. persicae placed in desiccators (12 L, Duran, Germany) sealed with glass stoppers containing a septum of filter paper for 20 h for PH3 at 20 °C. A 12-L desiccator (Bibby Scientific, Stone, UK, Cat. No. FE 12L/4) equipped with a lid fitted with a septum injection system (Alltech Crop Science, Nicholasville, KY, USA, Cat. No. 95326) was used for the fumigation of M. persicae. The volume of each desiccator was measured by weighing the amount of water at 20 °C. A magnetic bar was placed at the bottom of each desiccator to stir the gas and ensure even distribution of the fumigant.
2.4. Measurement of PH3 Concentrations
To monitor the fumigation concentration in the 12-L desiccator, 50-mL gas samples were drawn with a syringe from the chamber and stored in 1-L Tedlar® gas sampling bags (SKC, Dorset, UK) and analyzed, typically within 10 min of sampling. The concentration of PH3 was monitored at 10 min and 1, 3, 6, and 20 h. The subsequent concentration was determined using an Agilent GC 7890A equipped with a flame photometric detector (FPD) and HP-PLOT/Q (30 m × 530 µm × 40 µm; Agilent, Santa Clara, CA, USA) operating in split mode (10:1). The injector and oven temperatures were set to 200 °C. The detector temperature was 250 °C. The injection volume and flow rate were 20 µL and 5 mL/min, respectively. The concentrations of PH3 were calculated based on peak areas against external standards.
2.5. Determination of the Concentration × Time (CT) of Fumigants
The concentrations of the fumigants were monitored at timed intervals over the exposure period and used to calculate the CT (concentration × time) values using Equation (1).
where C is the concentration of the fumigant (mg/L); t is the time of exposure (h); i is the order of measurement; and CT is the concentration × time (mg h/L)
PH3 toxicity against M. persicae was described as the mortality of >30 M. persicae adults for at least three different CT values based on an average and SE (standard error) analysis. We calculated the concentration × time values for 10% (CT 10) and 50% mortality (CT 50), as well as the time values for 10% and 50% mortality due to PH3 and EF fumigation based on a Probit analysis using the SPSS statistics software (version 23.0).
2.6. Protein Extraction
Two groups of M. persicae were collected after fumigation assay of PH3, and immediately frozen at −70 °C. The frozen M. persicae (50 individuals) were homogenized with Tris-buffer containing 500 mM sucrose (pH 7.4) using pencil-type homogenizer. The homogenized solution was centrifuged at 600× g and 4 °C for 10 min. The supernatant (whole protein extract) was centrifuged at 10,000× g and 4 °C for 15 min, the pellet containing mitochondria was named “the mitochondrial fraction,” and the supernatant containing the soluble cytosolic portion was named “the S9 fraction”. Protein quantification was performed using the Bradford Assay with the Protein Assay Dye Reagent Concentrate (Bio-Rad, Hercules, CA, USA), and the protein standard curves were constructed using varying concentrations of BSA according to the manufacturer’s recommendations.
2.7. Enzyme Assay
The reduced cytochrome c was prepared as follows for use as the substrate of COX. Cytochrome c (2.7 mg) was weighed and dissolved in 1 mL of distilled water. To this solution, 5 μL of 0.1 M 1,4-Dithiothreitol (DTT) was added as a reducing agent and allowed to react at room temperature for 30 min until the color changed from dark brown to pale pink. The fully reduced cytochrome c solution was diluted 10-fold with Tris-buffer (120 mM KCl, 10 mM Tris-HCl, pH 7.4) and used as the reaction solution of COX. The mitochondrial fraction was properly diluted with Tris-buffer containing 500 mM sucrose (pH 7.4). To measure the activity of COX, 20 μL of the mitochondrial fraction containing 0.0012 mg of proteins and 180 μL of the reaction solution were mixed in a 96-well plate at 25 °C and measured at 550 nm for 30 min at 30 s intervals.
Acetylcholinesterase (AChE) activity was determined using the Ellman method [
15] at 412 nm. These enzyme activities were measured using the S9 fraction as described above. Enzyme activities were expressed in units/mg, meaning one unit would react with 1 μM substrate per min at 25 °C and pH 7.4. The data were expressed as mean ± standard deviation (SD) and analyzed using a one-way analysis of variance (ANOVA) and Tukey’s test as a post-hoc test in SPSS (version 23.0). All experiments were independently performed in triplicate.
2.8. RNA Extraction and RT-qPCR
M. persicae (50 individuals) in two groups were independently collected and frozen immediately at −70 °C.
M. persicae were rinsed twice with DEPC-treated water and homogenized using pencil-type homogenizer. The DEPC-treated water was purchased from Biosesang (Seongnam, Korea). To the homogenized sample, 1 mL of QIAzol lysis reagent (Qiagen, Düsseldorf, Germany) was added and incubated on ice for 20 min. The total RNA of each
M. persicae group was extracted according to the manufacturer’s protocol. The quality of total RNA was determined by measuring its A260/280 nm ratio (1.8–2.0) and checked by agarose gel electrophoresis. The Complementary DNA (cDNA) was immediately synthesized using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Fisher Scientific Inc., Waltham, MA, USA) and stored at −20 °C. A quantitative PCR (qPCR) was performed with a CYBR
® Green kit using Rotor-gene
® Q (Qiagen, Düsseldorf, Germany) following the manufacturer’s recommendations: 95 °C for 10 min (hold), followed by 40 cycles of 95 °C for 10 s, 60 °C for 15 s, and 72 °C for 20 s, and then an increase of 1 °C from 72 °C to 95 °C (melt). The primers for
M. persicae were designed using Primer-BLAST (Ye et al., 2012) and are listed in
Table S1. The beta-actin (
actb) and ribosomal protein S2 (
rps2) were used to normalize the expression level of the gene of interest (GOI). The results of RT-qPCR were expressed using the ∆∆Ct method [
16] and analyzed using a one-way ANOVA and Tukey’s test as a post-hoc test using the SPSS statistics software (version 23.0). All experiments were independently performed in triplicate.
2.9. Protein Preparation for Proteomics
The whole protein extract containing the mitochondrial fraction was obtained from
M. persicae, following the same procedure mentioned in
Section 2.6. The Tris-buffer containing 500 mM sucrose (pH 7.4) and Protease Inhibitor Cocktail Set I (Calbiochem, San Diego, CA, USA) was used to avoid protein degradation during protein extraction. The protein extracts were denatured by incubating them with 50 mM ammonium bicarbonate buffer (pH 7.8) containing 6 M Urea for 3 h at room temperature. The denatured protein extracts were incubated with 10 mM dithiothreitol for 2 h at room temperature to reduce their disulfide bonds. Subsequently, the reduced protein extracts were reacted first with iodoacetamide (IAA) for 1 h and then with trypsin at 37 °C in a shacking incubator for 18 h. The reacted samples were desalted using the Sep-Pac C18 cartridges (Waters Co., Milford, MA, USA) according to the manufacturer’s protocol.
2.10. Proteomic Analysis Using a Nano-LC-ESI-MS/MS
A Thermo Scientific Q Exactive Hybrid Quadrupole-Orbitrap (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with Dionex U 3000 RSLCnano HPLC system was used for proteomic analyses. The mass spectrometric analyses were performed with a nano-electrospray ionization source (ESI) and a fused silica emitter tip (New Objective, Woburn, MA, USA). The water/acetonitrile (98:2 v/v) solution containing 0.1% formic acid was used as the aqueous mobile phase. The samples were trapped on an Acclaim PepMap 100 trap column (100 μm × 2 cm, nanoViper C18, 5 μm, 100 Å) and washed for 6 min at a flow rate of 4 μL/min and then separated on an Acclaim PepMap 100 capillary column (75 μm × 15 cm, nanoViper C18, 3 μm, 100 Å) at a flow rate of 300 mL/min. The resulting peptides were electro-sprayed through a coated silica tip at an ion spray voltage of 2000 eV. The mass data were analyzed using Proteome Discoverer 1.4, MaxQuant 1.6, and Scaffold 4.8.4 against the protein databases for M. persicae. Significant differences between two groups of data were obtained using Student’s t-test (p < 0.05).
4. Discussion
PH
3 toxicity has been widely studied using its target invertebrates, including stored products insect pests, to understand how it disrupts normal physiological functions, leading to death [
10,
11,
12]. A recent paper by Nath et al. [
17] reviewed the two major routes of PH
3 toxicity; an energy imbalance via the breakdown of the electron transport system in mitochondria, and metabolic disturbance. Other toxic mechanisms by which PH
3 acts have been identified in rats and other mammals, such as a neurological disorder via potent inhibition on AChE activity, and severe inhibitory effects on metalloproteins, such as catalases and COX [
18].
Prior to an amassing a deeper understanding of PH
3 toxicity in
M. persicae, many studies showed that PH
3 interfered with COX activity, which is known as complex III in the electron transport chain [
18,
19]. Cytochrome c cox is a hemeprotein, within which PH
3 can easily form coordination bonds with the ferric ions, thereby reducing COX activity to produce less ATP than normal [
18].
In our study,
M. persicae was exposed to low PH
3 concentrations as LCT
10 value (LC
10 value), which did not kill
M. persicae. As shown in
Figure 2, in the PH
3-treated adults
M. persicae COX activity decreased with increasing concentrations of PH
3. However, this does not indicate that a direct inhibition of PH
3 on the target site of COX occurred or there was an indirect inhibition of COX activity by PH
3. LC-MS/MS analysis showed that COX expression decreased after PH
3 treatment (
Figure 4A). Therefore, a reduction in COX activity after PH
3 treatment was related to the reduced expression of the corresponding protein in
M. persicae. This result was confirmed by Chaudhry and Price [
18], who showed that no spectral changes with COX were found after the PH
3 treatment, indicating that there was no direct or weak contact between the COX protein and PH
3 molecules. On the contrary, other hemeproteins, hemoglobin, and myoglobin showed slow deoxygenation with spectral changes after PH
3 treatment [
18].
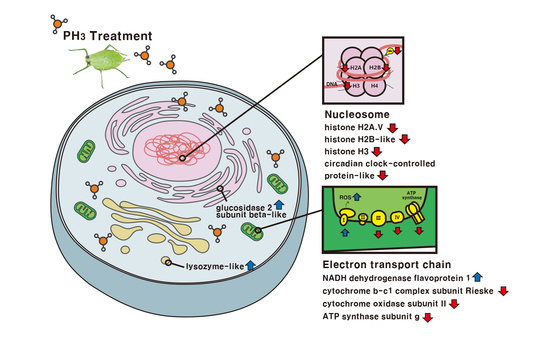
Interestingly, changes in COX activity were not dramatic, whereas cytochrome b-c1 complex subunit Rieske and ATP synthase subunit g in the PH
3-treated
M. persicae decreased by around 0.5-fold in terms of protein expression when compared with the control. Similarly, a reduction in ATP synthase was confirmed by Liu et al. [
20], which showed a 0.15-fold change in PH
3-treated larvae of the peach fruit moth,
Carposina sasakii Matsumura, using 2-dimesional electrophoresis with the MALDI-TOF MS analysis.
In this study, the most important finding is the dramatic change in NADH dehydrogenase (ubiquinone) flavoprotein 1 (×3.35-fold), which is complex I in the electron transport chain. It may be the first evidence that this protein is involved in PH3 toxicity in M. persicae. The ETC in the PH3-treated M. persicae was largely modified and the imbalance in ATP production was probably related to the metabolic disorder, leading to death of M. persicae.
However, regarding the up-regulation of NADH dehydrogenase (ubiquinone) flavoprotein 1 (complex I), one possible cause of PH
3 toxicity may be the overproduction of reactive oxygen species (ROS). As ROS are primarily generated in the mitochondrial ETC, complexes I and III are major sites for the production of small quantities of ROS [
20,
21]. Normally, the generated ROS is converted to hydrogen peroxide by superoxide dismutase, and then hydrogen peroxide can be transformed to water by peroxidases, which PH
3 inhibits in
Sitophilus granarius [
22]. Therefore, more ROS formation will probably occur in response to the up-regulation of complex I after PH
3 fumigation, and this ROS generation can lead to the death of
M. persicae during PH
3 fumigation. In addition to this oxidative stress, one of the regulatory proteins, ion protease homolog in mitochondria, was down-regulated and it is involved in maintaining protein quality and controlling the occurrence of misfolded or incompletely synthesized proteins (
Figure 4A). We need to further study the role of proteins in PH
3 toxicity in
M. persicae.
During PH
3 treatment,
M. persicae experienced induced expression of defense-related proteins including glutathione S-transferase-like (×2.26-fold), lysozyme-like (×3.14-fold), probable protein phosphatase 2C 6 (×2.02-fold), and a cluster of esterase E4-like isoform X1 (
Figure 4B). These are involved in chemical detoxification (glutathione S-transferase-like and Cluster of esterase E4-like isoform X1) and antimicrobial properties (lysozyme-like and probable protein phosphatase 2C 6). Glutathione S-transferase-like and a cluster of esterase E4-like isoform X1 are easily found in organophosphorus insecticides (OP insecticides)-resistant
M. persicae [
23]. Therefore, their up-regulation in PH
3-treated
M. persicae is related to the chemical structure of PH
3 and this includes the phosphorus ion. However, they may not be involved with the actual mechanism of PH
3 detoxification in the insect because PH
3 does not have an ester bond for esterase or an electrophilic site for conjugation reaction by glutathione
S-transferase.
However, there was no relationship between the inhibition of AChE activity and PH
3 treatment in
M. persicae in our study (
Figure 2), even though PH
3 inhibited AChE activity in
Ephestia cautella (Lepidoptera: Pyralidae) [
22]. This difference may be due to the low (equivalent to LCT10) concentration of PH
3 in the treatments. Al-Hakkak et al. [
24] used a series of concentrations of PH
3 treatments, which could kill the pupae of
Ephestia cautella. This phenomenon has been corroborated by Nayak and Collins [
25], who showed that PH
3 toxicity was influenced by concentration, in a study involving fumigation of
Liposcelis bostrychophila. Therefore, at a low PH
3 concentration, AChE activity will not be targeted in insects. Other neurologically related proteins, including neurotransmitter receptors and channels, were not affected by PH
3 fumigation (
Figure 3).
At low PH
3 concentrations, various regulation signals such as synaptic vesicle membrane protein VAT-1 (negative regulation of mitochondrial fusion), V-type proton ATPase subunit d (regulation of macroautophagy), serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform (positive regulation of apoptotic process), cathepsin B (regulation of apoptotic process), and slit homolog 1 protein-like (positive regulation of apoptotic process, response to cortisol), were turned on in
M. persicae (
Figure 4 and
Figure 5). This suggests that even low PH
3 concentrations induce the apoptotic process in
M. persicae.
Finally, metabolic changes in relation to PH
3 toxicity were also observed with the up-regulation of glucosidase2 subunit beta-like (×10.3-fold) and galactokinase-like (×2.20-fold), similar to findings by Nath et al. [
17]. This may be the first study to show such dramatic increases in glucosidase in relation to PH
3 toxicity.