Comparison of the Effects of Ketorolac and Acetaminophen on RANK-L Levels in the Gingival Crevicular Fluid during Orthodontic Tooth Movement: A Pilot Study
Abstract
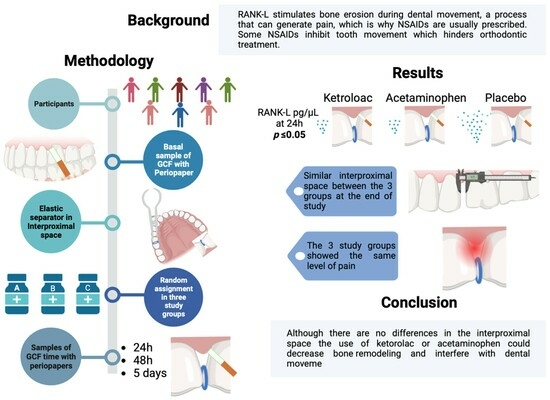
:1. Introduction
2. Materials and Methodology
2.1. Selection Criteria
2.2. Study Groups
2.3. Determination of Clinical and Demographic Variables
2.4. Sample Collection
2.5. Sample Preparation
2.6. Enzyme-Linked Immunosorbent Assay ELISA
2.7. Statistical Analysis
3. Results
3.1. Clinical and Sociodemographic Data
3.2. RANK-L Levels at the Four Sampling Times
3.3. RANK-L Levels between Study Groups
3.4. Correlation of the Different Study Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, R.R.; Nayme, J.G.; Garbin, A.J.; Saliba, N.; Garbin, C.A.; Moimaz, S.A. Prevalence of malocclusion and related oral habits in 5-to 6-year-old children. Oral. Health Prev. Dent. 2012, 10, 311–318. [Google Scholar]
- Lombardo, G.; Vena, F.; Negri, P.; Pagano, S.; Barilotti, C.; Paglia, L.; Colombo, S.; Orso, M.; Cianetti, S. Worldwide prevalence of malocclusion in the different stages of dentition: A systematic review and meta-analysis. Eur. J. Paediatr. Dent. 2020, 21, 115–122. [Google Scholar] [CrossRef]
- Amr-Rey, O.; Sanchez-Delgado, P.; Salvador-Palmer, R.; Cibrian, R.; Paredes-Gallardo, V. Association between malocclusion and articulation of phonemes in early childhood. Angle Orthod. 2022, 92, 505–511. [Google Scholar] [CrossRef]
- Santana, L.G.; Flores-Mir, C.; Iglesias-Linares, A.; Pithon, M.M.; Marques, L.S. Influence of heritability on occlusal traits: A systematic review of studies in twins. Prog. Orthod. 2020, 21, 29. [Google Scholar] [CrossRef]
- Thomas, M. Orthodontics in the “Art” of Aesthetics. Int. J. Orthod. 2015, 26, 23–28. [Google Scholar]
- Davidovitch, Z. Tooth movement. Crit. Rev. Oral. Biol. Med. 1991, 24, 411–450. [Google Scholar] [CrossRef]
- Lekic, P.; McCulloch, C.A. Periodontal ligament cell population: The central role of fibroblasts in creating a unique tissue. Anat. Rec. 1996, 2, 327–341. [Google Scholar] [CrossRef]
- Terai, K.; Takano-Yamamoto, T.; Ohba, Y. Role of osteopontin in bone remodeling caused by mechanical stress. J. Bone Miner. Res. 1999, 6, 839–849. [Google Scholar] [CrossRef]
- Pavlin, D.; Gluhak-Heinrich, J. Effect of mechanical loading on periodontal cells. Crit. Rev. Oral. Biol. Med. 2001, 5, 414–424. [Google Scholar] [CrossRef]
- Wise, G.E.; King, G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 5, 414–434. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, H.; Gong, Y. Mechanical strain promotes osteoblastic differentiation through integrin-β1-mediated β-catenin signaling. Int. J. Mol. Med. 2016, 2, 594–600. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Nicolay, O.F.; Ngan, P.W.; Shanfeld, J.L. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent. Clin. N. Am. 1988, 32, 411–435. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Alhashimi, N.; Frithiof, L.; Brudvik, P.; Bakhiet, M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 307–312. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Monk, A.B.; Harrison, J.E.; Worthington, H.V.; Teague, A.; Ertan Erdinç, A.M.; Dinçer, B.; Zhang, F.; Li, F.; Yang, H.; Jin, Y.; et al. Perception of Pain during Orthodontic Treatment with Fixed Appliances. Cochrane Database Syst. Rev. 2022, 26, 79–85. [Google Scholar]
- Monk, A.B.; Harrison, J.E.; Worthington, H.V.; Teague, A. Pharmacological interventions for pain relief during orthodontic treatment. Cochrane Database Syst. Rev. 2017, 11, CD003976. [Google Scholar] [CrossRef]
- Huang, H.; Williams, R.C.; Kyrkanides, S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am. J. Orthod. Dentofacial Orthop. 2014, 146, 620–632. [Google Scholar] [CrossRef]
- Wang, Y.H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.J.; Dong, C.; Homey, B.; et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007, 204, 1837–1847. [Google Scholar] [CrossRef]
- Çelebi, F. Mechanical Vibration and Chewing Gum Methods in Orthodontic Pain Relief. Turk. J. Orthod. 2022, 35, 133–138. [Google Scholar] [CrossRef]
- Cheng, C.; Xie, T.; Wang, J. The efficacy of analgesics in controlling orthodontic pain: A systematic review and meta-analysis. BMC Oral. Health 2020, 20, 259. [Google Scholar] [CrossRef]
- Meier, M.; Bauer, K.; Proff, P.; Fanghänel, J. Meloxicam medication reduces orthodontically induced dental root resorption and tooth movement velocity: A combined in vivo and in vitro study of dental-periodontal cells and tissue. Cell Tissue Res. 2017, 368, 61–78. [Google Scholar] [CrossRef]
- Kaku, M.; Yamamoto, T.; Yashima, Y.; Izumino, J.; Kagawa, H.; Ikeda, K.; Tanimoto, K. Acetaminophen reduces apical root resorption during orthodontic tooth movement in rats. Arch. Oral. Biol. 2019, 102, 83–92. [Google Scholar] [CrossRef]
- Alshammari, A.K.; Huggare, J. Pain relief after orthodontic archwire installation-a comparison between intervention with paracetamol and chewing gum: A randomized controlled trial. Eur. J. Orthod. 2019, 41, 478–485. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W.; Larson, B.; Sarver, D.M. Ortodoncia Contemporánea; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; ISBN 849113591X. [Google Scholar]
- Roy, J.; Dempster, L.J. Dental anxiety associated with orthodontic care: Prevalence and contributing factors. Semin. Orthod. 2018, 24, 233–241. [Google Scholar] [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod. Craniofac Res. 2006, 9, 63–70. [Google Scholar] [CrossRef]
- Shetty, N.; Patil, A.K.; Ganeshkar, S.V.; Hegde, S. Comparison of the effects of ibuprofen and acetaminophen on PGE2 levels in the GCF during orthodontic tooth movement: A human study. Prog. Orthod. 2013, 14, 6. [Google Scholar] [CrossRef]
- Amanda, J.; Widayati, R.; Soedarsono, N.; Purwanegara, M.K. RANKL Concentrations in Early Orthodontic Treatment Using Passive Self-Ligating and Preadjusted Edgewise Appliance Bracket Systems. J. Phys. Conf. Ser. 2018, 1073, 042002. [Google Scholar] [CrossRef]
- Celebi, F.; Bicakci, A.A.; Kelesoglu, U. Effectiveness of low-level laser therapy and chewing gum in reducing orthodontic pain: A randomized controlled trial. Korean J. Orthod. 2021, 51, 313–320. [Google Scholar] [CrossRef]
- Sudhakar, V.; Vinodhini, T.S.; Mohan, A.M.; Srinivasan, B.; Rajkumar, B.K. The efficacy of different pre- and post-operative analgesics in the management of pain after orthodontic separator placement: A randomized clinical trial. J. Pharm. Bioallied. Sci. 2014, 6 (Suppl. 1), S80–S84. [Google Scholar] [CrossRef]
- Chow, J.; Cioffi, I. Pain and orthodontic patient compliance: A clinical perspective. Semin. Orthod. 2018, 24, 242–247. [Google Scholar] [CrossRef]
- Kaur, H.; Bansal, N.; Abraham, R. A randomized, single-blind, placebo-controlled trial to evaluate the effectiveness of verbal behavior modification and acetaminophen on orthodontic pain. Angle Orthod. 2019, 89, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Cossellu, G.; Lanteri, V.; Lione, R.; Ugolini, A.; Gaffuri, F.; Cozza, P.; Farronato, M. Efficacy of ketoprofen lysine salt and paracetamol/acetaminophen to reduce pain during rapid maxillary expansion: A randomized controlled clinical trial. Int. J. Paediatr. Dent. 2019, 29, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Salmassian, R.; Oesterle, L.J.; Shellhart, W.C.; Newman, S.M. Comparison of the efficacy of ibuprofen and acetaminophen in controlling pain after orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 516–521. [Google Scholar] [CrossRef]
- Owayda, A.M.; Hajeer, M.Y.; Murad, R.M.T.; Al-Sabbagh, R. The efficacy of low-level laser therapy versus paracetamol-caffeine in controlling orthodontic separation pain and changes in the oral-health-related quality of life in Class I malocclusions: A 3-arm, randomized, placebo-controlled clinical trial. J. World Fed. Orthod. 2022, 11, 75–82. [Google Scholar] [CrossRef]
- Yassaei, S.; Vahidi, A.; Farahat, F. Comparison of the efficacy of calcium versus acetaminophen on reduction of orthodontic pain. Indian J. Dent. Res. 2012, 23, 608–612. [Google Scholar] [CrossRef]
- Tunçer, Z.; Polat-Ozsoy, O.; Demirbilek, M.; Bostanoglu, E. Effects of various analgesics on the level of prostaglandin E2 during orthodontic tooth movement. Eur. J. Orthod. 2014, 36, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.; Perretti, M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: Two enzymes in the spotlight. Biochem. Pharmacol. 2003, 65, 153–159. [Google Scholar] [CrossRef]
- Aranda, J.V.; Salomone, F.; Valencia, G.B.; Beharry, K.D. Non-steroidal anti-inflammatory drugs in newborns and infants. Pediatr. Clin. N. Am. 2017, 64, 1327–1340. [Google Scholar] [CrossRef]
- Henneman, S.; Von den Hoff, J.W.; Maltha, J.C. Mechanobiology of tooth movement. Eur. J. Orthod. 2008, 30, 299–306. [Google Scholar] [CrossRef]
- Hammad, S.M.; El-Hawary, Y.M.; El-Hawary, A.K. The use of different analgesics in orthodontic tooth movements. Angle Orthod. 2012, 82, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Muth-Selbach, U.S.; Tegeder, I.; Brune, K.; Geisslinger, G. Acetaminophen inhibits spinal prostaglandin E2 release after peripheral noxious stimulation. Anesthesiology 1999, 91, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S.; Colville-Nash, P.R.; Willoughby, D.A.; Botting, R.M. The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice. Eur. J. Pharmacol. 2006, 538, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Akarasereenont, P.; Thiemermann, C.; Flower, R.J.; Vane, J.R. Selectivity of non-steroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl. Acad. Sci. USA 1993, 90, 11693–11697. [Google Scholar] [CrossRef]
- Botting, R.M. Mechanism of Action of Acetaminophen: Is There a Cyclooxygenase 3? Clin. Infect. Dis. 2000, 31, S202–S210. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Karthi, M.; Anbuslevan, G.J.; Senthilkumar, K.P.; Tamizharsi, S.; Raja, S.; Prabhakar, K. NSAIDs in orthodontic tooth movement. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S2), S304–S306. [Google Scholar] [CrossRef] [PubMed]
- Monk, A. Pharmacological Interventions for Pain Relief during Orthodontic Treatment. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 2016. [Google Scholar]
- Hosseinzadeh Nik, T.; Shahsavari, N.; Ghadirian, H.; Ostad, S.N. Acetaminophen Versus Liquefied Ibuprofen for Control of Pain During Separation in Orthodontic Patients: A Randomized Triple Blinded Clinical Trial. Acta Med. Iran. 2016, 54, 418–421. [Google Scholar]
- Shen, H.; Shao, S.; Zhang, J.; Wang, Z.; Lv, D.; Chen, W.; Svensson, P.; Wang, K. Fixed orthodontic appliances cause pain and disturbance in somatosensory function. Eur. J. Oral Sci. 2016, 124, 26–32. [Google Scholar] [CrossRef]
- Kawasaki, K.; Takahashi, T.; Yamaguchi, M.; Kasai, K. Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod. Craniofac Res. 2006, 9, 137–142. [Google Scholar] [CrossRef]
- Tripathi, T.; Singh, N.; Rai, P.; Khanna, N. Separation and pain perception of Elastomeric, Kesling and Kansal separators. Dental Press J. Orthod. 2019, 24, 42–48. [Google Scholar] [CrossRef]
- Davidovitch, M.; Papanicolaou, S.; Vardimon, A.D.; Brosh, T. Duration of elastomeric separation and effect on interproximal contact point characteristics. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Asiry, M.A.; Albarakati, S.F.; Al-Marwan, M.S.; Al-Shammari, R.R. Perception of pain and discomfort from elastomeric separators in Saudi adolescents. Saudi Med. J. 2014, 35, 504–507. [Google Scholar] [PubMed]
- Al-Balbeesi, H.O.; Bin Huraib, S.M.; AlNahas, N.W.; AlKawari, H.M.; Abu-Amara, A.B.; Vellappally, S.; Anil, S. Pain and distress induced by elastomeric and spring separators in patients undergoing orthodontic treatment. J. Int. Soc. Prev. Community Dent. 2016, 6, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Zarif Najafi, H.; Oshagh, M.; Salehi, P.; Babanouri, N.; Torkan, S. Comparison of the effects of preemptive acetaminophen, ibuprofen, and meloxicam on pain after separator placement: A randomized clinical trial. Prog. Orthod. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed]



| Control (n = 8) | Ketorolac (n = 8) | Acetaminophen (n = 8) | p | |
|---|---|---|---|---|
| Male | 2 (25) | 1 (12.5) | 5 (62.5) | 0.087 |
| Female | 6 (75) | 7 (87.5) | 3 (37.5) | |
| Age (years) | 21.75 ± 1.55 | 21.13 ± 1.61 | 20.88 ± 1.35 | 0.884 |
| Molar Class I | 6 (75) | 7 (87.5) | 7 (87.5) | 0.741 |
| Molar Class II | 2 (25) | 1 (12.5) | 1 (12.5) | |
| Pain | 2 (25) | 3 (37.5) | 0 (0) | 0.304 |
| No pain | 6 (76) | 5 (62.5) | 8 (100) | |
| VAS | 0.42 ± 0.42 | 1.62 ± 0.84 | 0 ± 0 | 0.634 |
| Chewing discomfort | 0 (0) | 1 (12.5) | 1 (12.5) | 1.000 |
| No chewing discomfort | 8 (100) | 7 (87.5) | 7 (87.5) | |
| Flu | 1 (12.5) | 0 (0) | 0 (0) | |
| No flu | 7 (87.5) | 8 (100) | 8 (100) | 1.000 |
| Headache | 1 (12.5) | 1 (12.5) | 0 (0) | |
| No headache | 7 (87.5) | 7 (87.5) | 8 (100) | 1.000 |
| Heartburn | 1 (12.5) | 1 (12.5) | 0 (0) | |
| No heartburn | 7 (87.5) | 7 (87.5) | 8 (100) | 1.000 |
| Interproximal space (mm) | 0.28 (0.27) | 0.26 (0.09) | 0.28 (0.09) | 0.632 |
| Sample Time | Control pg/µL | Ketorolac pg/µL | Acetaminophen pg/µL | p | |
|---|---|---|---|---|---|
| T0 | M (IQR) ± SD | 0.032 (0.049) 0.225 ± 0.527 | 0.031 (0.008) 0.113 ± 0.236 | 0.032 (0.006) 0.039 ± 0.006 | 0.665 |
| T1 | M (IQR) ± SD | 0.038 (0.028) 0.146 ± 0.278 | 0.029 (0.024) 0.036 ± 0.021 | 0.030 (0.018) 0.047 ± 0.052 | 0.050 * |
| T2 | M (IQR) ± SD | 0.033 (0.009) 0.350 ± 0.849 | 0.032 (0.008) 0.046 ± 0.039 | 0.033 (0.028) 0.042 ± 0.018 | 0.935 |
| T3 | M (IQR) ± SD | 0.035 (0.159) 0.111 ± 0.118 | 0.030 (0.012) 0.188 ± 0.446 | 0.035 (0.039) 0.041 ± 0.023 | 0.228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Montaño, R.; Ponce-Gómez, Y.I.; Lomelí-Martínez, S.M.; Sifuentes-Franco, S.; Ruiz-Gutiérrez, A.d.C.; Bayardo-González, R.A.; Martínez-Rodríguez, V.M.d.C.; Meléndez-Ruíz, J.L.; Gómez-Sandoval, J.R. Comparison of the Effects of Ketorolac and Acetaminophen on RANK-L Levels in the Gingival Crevicular Fluid during Orthodontic Tooth Movement: A Pilot Study. Appl. Sci. 2024, 14, 1464. https://doi.org/10.3390/app14041464
Rodríguez-Montaño R, Ponce-Gómez YI, Lomelí-Martínez SM, Sifuentes-Franco S, Ruiz-Gutiérrez AdC, Bayardo-González RA, Martínez-Rodríguez VMdC, Meléndez-Ruíz JL, Gómez-Sandoval JR. Comparison of the Effects of Ketorolac and Acetaminophen on RANK-L Levels in the Gingival Crevicular Fluid during Orthodontic Tooth Movement: A Pilot Study. Applied Sciences. 2024; 14(4):1464. https://doi.org/10.3390/app14041464
Chicago/Turabian StyleRodríguez-Montaño, Ruth, Yesenia Isahy Ponce-Gómez, Sarah Monserrat Lomelí-Martínez, Sonia Sifuentes-Franco, Alondra del Carmen Ruiz-Gutiérrez, Rubén Alberto Bayardo-González, Vianeth María del Carmen Martínez-Rodríguez, José Luis Meléndez-Ruíz, and Juan Ramón Gómez-Sandoval. 2024. "Comparison of the Effects of Ketorolac and Acetaminophen on RANK-L Levels in the Gingival Crevicular Fluid during Orthodontic Tooth Movement: A Pilot Study" Applied Sciences 14, no. 4: 1464. https://doi.org/10.3390/app14041464