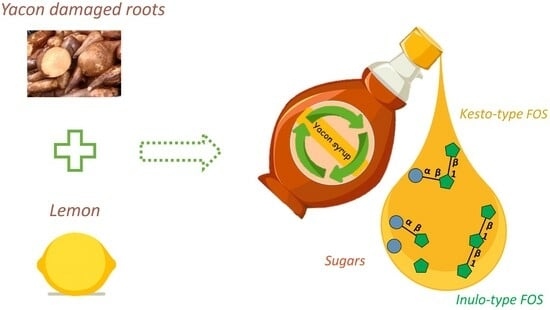
Utilization of Yacon Damaged Roots as a Source of FOS-Enriched Sweet-Tasting Syrup
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hydroethanolic Extractions
2.3. Syrup Production
2.4. Chemical Composition
2.4.1. Moisture and Total Solids Content
2.4.2. Carbohydrate Composition
2.4.3. Protein Analysis
2.5. Texture Analysis
2.6. Syrups Sweetness and Nutritional Features
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yacon Varieties Composition
3.2. Syrup Production
3.2.1. Effect of Citric Acid Concentration on Syrup Properties
3.2.2. Effect of Ascorbic Concentration on Syrup Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caetano, B.F.R.; De Moura, N.A.; Almeida, A.P.S.; Dias, M.C.; Sivieri, K.; Barbisan, L.F. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Kamp, L.; Hartung, J.; Mast, B.; Graeff-Hönninger, S. Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe. Agronomy 2019, 9, 301. [Google Scholar] [CrossRef]
- Reis, F.R.; Marques, C.; Moraes, A.C.S.d.; Masson, M.L. Effect of processing methods on yacon roots health-promoting compounds and related properties. Trends Food Sci. Technol. 2021, 113, 346–354. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) flour soluble extract improve intestinal bacterial populations, brush border membrane functionality and morphology in vivo (Gallus gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Caballero, M.; Herbas, A.; Carballo, S. Antioxidants in yacon products and effect of long term storage. Food Sci. Technol. 2012, 32, 432–435. [Google Scholar] [CrossRef]
- Marques, C.; Bortolan Toazza, C.E.; Sari, R.; Mitterer-Daltoé, M.L.; Amaral, W.D.; Masson, M.L. Long-term storage of yacon (Smallanthus sonchifolius) juice: Phytochemical profile, in vitro prebiotic potential and discriminant bioactive properties. Food Biosci. 2021, 41, 100970. [Google Scholar] [CrossRef]
- World Health Organization. Taxes on Sugary Drinks: Why Do It? World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gomes, A.; Bourbon, A.I.; Peixoto, A.R.; Silva, A.S.; Tasso, A.; Almeida, C.; Nobre, C.; Nunes, C.; Sánchez, C.; Gonçalves, D.A.; et al. Strategies for the reduction of sugar in food products. In Food Structure Engineering and Design for Improved Nutrition, Health and Well-Being; Cerqueira, M.Â.P.R., Pastrana Castro, L.M., Eds.; Academic Press: Massachusetts, MA, USA, 2023; pp. 219–241. [Google Scholar] [CrossRef]
- Castro, A.; Céspedes, G.; Carballo, S.; Bergenståhl, B.; Tornberg, E. Dietary fiber, fructooligosaccharides, and physicochemical properties of homogenized aqueous suspensions of yacon (Smallanthus sonchifolius). Food Res. Int. 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Paredes, L.L.R.; Smiderle, F.R.; Santana-Filho, A.P.; Kimura, A.; Iacomini, M.; Sassaki, G.L. Yacon fructans (Smallanthus sonchifolius) extraction, characterization and activation of macrophages to phagocyte yeast cells. Int. J. Biol. Macromol. 2018, 108, 1074–1081. [Google Scholar] [CrossRef]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular Structures of Fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef]
- Sales, S.d.S.; Dionísio, A.P.; Adriano, L.S.; Melo, B.R.C.d.; Abreu, F.A.P.d.; Sampaio, H.A.d.C.; Silva, I.D.C.G.d.; Carioca, A.A.F. Previous gut microbiota has an effect on postprandial insulin response after intervention with yacon syrup as a source of fructooligosaccharides: A randomized, crossover, double-blind clinical trial. Nutrition 2023, 109, 111948. [Google Scholar] [CrossRef] [PubMed]
- Choquechambi, L.A.; Callisaya, I.R.; Ramos, A.; Bosque, H.; Mújica, A.; Jacobsen, S.-E.; Sørensen, M.; Leidi, E.O. Assessing the Nutritional Value of Root and Tuber Crops from Bolivia and Peru. Foods 2019, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Roberfroid, M.R. Physiological Effects of Non-Digestible Oligosaccharides. LWT-Food Sci. Technol. 1994, 27, 1–6. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Knee, M. Polysaccharides and glycoproteins of apple fruit cell walls. Phytochemistry 1973, 12, 637–653. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Rodrigues, O.R.L.; Asquieri, E.R.; Orsi, D.C. Prevention of enzymatic browning of yacon flour by the combined use of anti-browning agents and the study of its chemical composition. Food Sci. Technol. 2014, 34, 275–280. [Google Scholar] [CrossRef]
- Manrique, I.; Parraga, A.; Hermann, M. Yacon Syrup: Principles and Processing; Series: Conservación y uso de la Biodiversidad de Raíces y Tubérculos Andinos: Una década de Investigación para el Desarrollo. (1993–2003); No. 8B; International Potato Center, Universidad Nacional Daniel Alcides Carrión, Erbacher Foundation, Swiss Agency for Development and Cooperation: Lima, Peru, 2005; pp. 1–31. [Google Scholar]
- Fernandes, P.A.R.; Silva, A.M.S.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The hydrophobic polysaccharides of apple pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J.; Ellis, P.R. The role of sugars and sweeteners in food, diet and health: Alternatives for the future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Tiefenbacher, K.F. Chapter Three—Technology of Main Ingredients—Sweeteners and Lipids. In Wafer and Waffle; Tiefenbacher, K.F., Ed.; Academic Press: Massachusetts, MA, USA, 2017; pp. 123–225. [Google Scholar] [CrossRef]
- Moskowitz, H.R. Ratio scales of sugar sweetness. Percept. Psychophys. 1970, 7, 315–320. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Goldman, F. Sugar Substitute and Bulking Agent and Chocolate. U.S. Patent US20060088637A1, 27 April 2006. [Google Scholar]
- Godshall, M.A. The expanding world of nutritive and non-nutritive sweeteners. Sugar J. 2007, 69, 12–20. [Google Scholar]
- European Parliament. Regulation (EU) No. 1169/2011; The Council of the European Union, Ed.; Official Journal European Union: Brussels, Belgium, 2011; Volume 304, pp. 18–63. [Google Scholar]
- Graefe, S.; Hermann, M.; Manrique, I.; Golombek, S.; Buerkert, A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crops Res. 2004, 86, 157–165. [Google Scholar] [CrossRef]
- Khajehei, F.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules 2018, 23, 278. [Google Scholar] [CrossRef]
- Kamp, L.; Hartung, J.; Mast, B.; Graeff-Hönninger, S. Impact of Nitrogen Fertilization on Tuber Yield, Sugar Composition and Nitrogen Uptake of Two Yacon (Smallanthus sonchifolius Poepp. & Endl.) Genotypes. Agronomy 2019, 9, 151. [Google Scholar] [CrossRef]
- Leroy, G.; Grongnet, J.F.; Mabeau, S.; Corre, D.L.; Baty-Julien, C. Changes in inulin and soluble sugar concentration in artichokes (Cynara scolymus L.) during storage. J. Sci. Food Agric. 2010, 90, 1203–1209. [Google Scholar] [CrossRef]
- Bekers, M.; Grube, M.; Upite, D.; Kaminska, E.; Linde, R.; Scherbaka, R.; Danilevich, A. Carbohydrates in Jerusalem artichoke powder suspension. Nutr. Food Sci. 2007, 37, 42–49. [Google Scholar] [CrossRef]
- Cabezas, M.J.; Rabert, C.; Bravo, S.; Shene, C. Inulin and Sugar Contents in Helianthus tuberosus and Cichorium intybus Tubers: Effect of Postharvest Storage Temperature. J. Food Sci. 2002, 67, 2860–2865. [Google Scholar] [CrossRef]
- Hamdi, A.; Viera-Alcaide, I.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Muñoz, M.J.; Monje Moreno, J.M.; Jiménez-Araujo, A. Asparagus Fructans as Emerging Prebiotics. Foods 2023, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Waleckx, E.; Gschaedler, A.; Colonna-Ceccaldi, B.; Monsan, P. Hydrolysis of fructans from Agave tequilana Weber var. azul during the cooking step in a traditional tequila elaboration process. Food Chem. 2008, 108, 40–48. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Ávila-Fernández, Á.; Galicia-Lagunas, N.; Rodríguez-Alegría, M.E.; Olvera, C.; López-Munguía, A. Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chem. 2011, 129, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Silva, M.F.G.d.; Dionísio, A.P.; Abreu, F.A.P.; Brito, E.S.d.; Wurlitzer, N.J.; Silva, L.M.A.E.; Ribeiro, P.R.V.; Rodrigues, S.; Taniguchi, C.A.K.; Pontes, D.F. Evaluation of nutritional and chemical composition of yacon syrup using 1H NMR and UPLC-ESI-Q-TOF-MSE. Food Chem. 2018, 245, 1239–1247. [Google Scholar] [CrossRef]
- Adriano, L.S.; Dionísio, A.P.; Pinto de Abreu, F.A.; Wurlitzer, N.J.; Cordeiro de Melo, B.R.; Ferreira Carioca, A.A.; de Carvalho Sampaio, H.A. Acute postprandial effect of yacon syrup ingestion on appetite: A double blind randomized crossover clinical trial. Food Res. Int. 2020, 137, 109648. [Google Scholar] [CrossRef]
- Mohammed, F.; Sibley, P.; Guillaume, D.; Abdulwali, N. Chemical composition and mineralogical residence of maple syrup: A comprehensive review. Food Chem. 2022, 374, 131817. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, composition and health-protective properties of citrus honey: A review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Blecker, C.; Fougnies, C.; Van Herck, J.-C.; Chevalier, J.-P.; Paquot, M. Kinetic study of the acid hydrolysis of various oligofructose samples. J. Agric. Food Chem. 2002, 50, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.J.; Manley-Harris, M.; Field, R.J.; Parker, B.A. Kinetics of Formation of Di-d-fructose Dianhydrides during Thermal Treatment of Inulin. J. Agric. Food Chem. 2000, 48, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Transglycosylation reactions, a main mechanism of phenolics incorporation in coffee melanoidins: Inhibition by Maillard reaction. Food Chem. 2017, 227, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Margalli, N.A.; López, M.G. Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber var. azul. J. Agric. Food Chem. 2002, 50, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; Gerschenson, L.N. Ascorbic Acid Destruction in Sweet Aqueous Model Systems. LWT-Food Sci. Technol. 1997, 30, 567–572. [Google Scholar] [CrossRef]
- Rojas, A.M.; Gerschenson, L.N. Ascorbic acid destruction in aqueous model systems: An additional discussion. J. Sci. Food Agric. 2001, 81, 1433–1439. [Google Scholar] [CrossRef]
- Dennison, D.B.; Brawley, T.G.; Hunter, G.L.K. Rapid high-performance liquid chromatographic determination of ascorbic acid and combined ascorbic acid-dehydroascorbic acid in beverages. J. Agric. Food Chem. 1981, 29, 927–929. [Google Scholar] [CrossRef]





| Hualqui | Crespo | |||||||
|---|---|---|---|---|---|---|---|---|
| Skin | Pulp | Skin | Pulp | |||||
| ηroot (%) | 20 | 80 | 20 | 80 | ||||
| Fraction | EtRe | EtSn | EtRe | EtSn | EtRe | EtSn | EtRe | EtSn |
| ηEtPp (%) | 31 | 49 | 9 | 86 | 42 | 56 | 64 | 34 |
| Sugar | ||||||||
| Glc | - | 100 ± 1 | tr | 92 ± 7 | tr | 78 ± 3 | tr | 117 ± 13 |
| Fru | - | 243 ± 5 | tr | 272 ± 7 | tr | 212 ± 21 | tr | 240 ± 9 |
| Suc | - | 34 ± 0 | tr | 47 ± 2 | tr | 57 ± 0 | tr | 51 ± 1 |
| FOS (kesto type) | ||||||||
| DP3 | - | 23 ± 1 | tr | 43 ± 1 | tr | 24 ± 0 | tr | 22 ± 0 |
| DP4 | - | 29 ± 0 | tr | 40 ± 2 | tr | 31 ± 3 | 3 ± 0 | 38 ± 1 |
| DP5 | - | 29 ± 1 | tr | 37 ± 2 | tr | 42 ± 2 | 8 ± 0 | 55 ± 1 |
| DP6 | - | 16 ± 0 | tr | 21 ± 1 | tr | 33 ± 2 | 10 ± 0 | 53 ± 2 |
| DP7 | - | 13 ± 1 | tr | 15 ± 1 | tr | 32 ± 4 | 12 ± 0 | 50 ± 2 |
| DP8 | - | 12 ± 0 | tr | 11 ± 1 | 2 ± 0 | 32 ± 2 | 15 ± 1 | 45 ± 6 |
| DP9 | - | 10 ± 0 | 1 ± 0 | 9 ± 0 | 3 ± 0 | 33 ± 2 | 20 ± 2 | 40 ± 2 |
| FOS (Inulo type) | ||||||||
| DP2 | - | 3 ± 0 | tr | 4 ± 0 | tr | 12 ± 1 | tr | 15 ± 0 |
| DP3 | - | - | tr | 1 ± 0 | tr | 11 ± 1 | 1 ± 0 | 16 ± 0 |
| DP4 | - | - | tr | - | tr | 4 ± 0 | tr | 8 ± 0 |
| DP5 | - | - | tr | - | tr | tr | - | - |
| DP6 | - | - | tr | - | tr | tr | - | - |
| Inulin | ||||||||
| DP10 | - | 7 ± 0 | 1 ± 0 | 6 ± 0 | 5 ± 1 | 31 ± 3 | 25 ± 1 | 36 ± 1 |
| DP11 | - | 5 ± 0 | 2 ± 0 | 4 ± 0 | 8 ± 1 | 27 ± 1 | 28 ± 0 | 29 ± 0 |
| DP12 | - | 4 ± 0 | 1 ± 0 | 2 ± 0 | 12 ± 0 | 23 ± 1 | 31 ± 2 | 21 ± 0 |
| DP13 | - | 3 ± 0 | 1 ± 0 | 1 ± 0 | 15 ± 1 | 18 ± 1 | 34 ± 2 | 17 ± 0 |
| DP14 | - | 2 ± 0 | 1 ± 0 | 1 ± 0 | 17 ± 2 | 13 ± 1 | 37 ± 1 | 15 ± 0 |
| DP15 | - | 1 ± 0 | 1 ± 0 | tr | 19 ± 2 | 9 ± 0 | 40 ± 1 | 9 ± 0 |
| DP16 | - | 1 ± 0 | tr | - | 20 ± 2 | 6 ± 1 | 34 ± 6 | 6 ± 0 |
| DP17 | - | 1 ± 0 | tr | - | 20 ± 2 | 3 ± 0 | 36 ± 1 | 4 ± 0 |
| DP18 | - | tr | - | - | 19 ± 1 | 2 ± 0 | 31 ± 4 | 3 ± 0 |
| DP19 | - | Tr | - | - | 18 ± 2 | 1 ± 0 | 30 ± 1 | 2 ± 0 |
| DP20 | - | - | - | - | 16 ± 1 | 1 ± 0 | 28 ± 0 | 1 ± 0 |
| DP21 | - | - | - | - | 15 ± 1 | tr | 24 ± 1 | 1 ± 0 |
| DP22 | - | - | - | - | 14 ± 1 | tr | 22 ± 1 | tr |
| DP23 | - | - | - | - | 12 ± 1 | - | 19 ± 0 | - |
| DP24 | - | - | - | - | 11 ± 1 | - | 17 ± 0 | - |
| DP25 | - | - | - | - | 10 ± 1 | - | 15 ± 0 | - |
| DP26 | - | - | - | - | 9 ± 0 | - | 13 ± 0 | - |
| DP27 | - | - | - | - | 8 ± 0 | - | 11 ± 0 | - |
| DP28 | - | - | - | - | 7 ± 0 | - | 10 ± 0 | - |
| DP29 | - | - | - | - | 6 ± 0 | - | 8 ± 0 | - |
| DP30 | - | - | - | - | 6 ± 0 | - | 7 ± 0 | - |
| DP31 | - | - | - | - | 5 ± 0 | - | 6 ± 0 | - |
| DP32 | - | - | - | - | 4 ± 0 | - | 5 ± 0 | - |
| DP33 | - | - | - | - | 4 ± 0 | - | 4 ± 0 | - |
| DP34 | - | - | - | - | 3 ± 0 | - | 4 ± 0 | - |
| DP35 | - | - | - | - | 3 ± 0 | - | 3 ± 0 | - |
| DP36 | - | - | - | - | 3 ± 0 | - | 3 ± 0 | - |
| DP37 | - | - | - | - | 2 ± 0 | - | 2 ± 0 | - |
| DP38 | - | - | - | - | 2 ± 0 | - | 2 ± 0 | - |
| DP39 | - | - | - | - | 2 ± 0 | - | 2 ± 0 | - |
| DP40 | - | - | - | - | 2 ± 0 | - | 1 ± 0 | - |
| DP41 | - | - | - | - | 1 ± 0 | - | 1 ± 0 | - |
| DP42 | - | - | - | - | 1 ± 0 | - | 1 ± 0 | - |
| DP43 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP44 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP45 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP46 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP47 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP48 | - | - | - | - | 1 ± 0 | - | tr | - |
| DP49 | - | - | - | - | tr | - | tr | - |
| DP50 | - | - | - | - | tr | - | tr | - |
| Total | - | 532 ± 10 | 9 ± 0 | 606 ± 8 | 311 ± 21 | 734 ± 41 | 608 ± 23 | 888 ± 17 |
| Variety | Fraction | Yield (%) | Carbohydrate Composition (molar %) | Total (g/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||||
| Hualqui | |||||||||||
| Skin | EtSn | 49 | - | - | 1 ± 0 | - | 22 ± 1 | - | 70 ± 2 | 7 ± 1 | 331 ± 37 |
| EtRe | 31 | tr | tr | 3 ± 0 | 1 ± 0 | tr | 1 ± 0 | 9 ± 1 | 86 ± 1 | 220 ± 9 | |
| Pulp | EtSn | 86 | - | - | - | - | 23 ± 1 | - | 71 ± 1 | 6 ± 2 | 402 ± 15 |
| EtRe | 9 | tr | - | 2 ± 0 | 1 ± 0 | tr | 1 ± 0 | 10 ± 2 | 84 ± 2 | 335 ± 11 | |
| Crespo | |||||||||||
| Skin | EtSn | 64 | - | - | - | - | 24 ± 1 | - | 71 ± 1 | 5 ± 0 | 538 ± 38 |
| EtRe | 34 | 1 ± 0 | 1 ± 0 | 13 ± 1 | 1 ± 0 | 22 ± 3 | 5 ± 0 | 48 ± 6 | 9 ± 1 | 338 ± 47 | |
| Pulp | EtSn | 56 | - | - | - | - | 28 ± 2 | - | 67 ± 1 | 5 ± 1 | 547 ± 26 |
| EtRe | 42 | tr | tr | 4 ± 0 | 1 ± 0 | 28 ± 1 | 2 ± 0 | 49 ± 1 | 15 ± 1 | 500 ± 16 | |
| Yacon Sample | Evaporation Pressure | Yield (%) * | °Brix | pH | TRS | TRG | Caloric Value (kcal/100 g) |
|---|---|---|---|---|---|---|---|
| Hualqui | |||||||
| Root | - | - | 6.7 | 6.03 | 0.51 ± 0.00 | 0.21 ± 0.01 | 157 ± 1 |
| Syrups | |||||||
| 5% lemon | Atmospheric | - | 72 | 4.15 | 0.88 ± 0.02 | 0.40 ± 0.01 | 256 ± 6 |
| 0.1% CitA | Atmospheric | 72 | 68 | 4.87 | 0.71 ± 0.01 | 0.30 ± 0.02 | 214 ± 4 |
| 0.25% CitA | Atmospheric | 70 | 70 | 4.20 | 0.89 ± 0.03 | 0.35 ± 0.01 | 263 ± 8 |
| 0.25% CitA + 0.08% AA | Atmospheric | 57 | 75 | 4.23 | 0.62 ± 0.00 | 0.30 ± 0.00 | 186 ± 2 |
| 0.5% CitA | Atmospheric | 79 | 68 | 3.90 | 0.91 ± 0.02 | 0.38 ± 0.00 | 253 ± 4 |
| Crespo | |||||||
| Root | - | - | 10.1 | 6.00 | 0.30 ± 0.01 | 0.12 ± 0.01 | 162 ± 5 |
| Syrups | |||||||
| 0.1% CitA | Atmospheric | 79 | 73 | 5.12 | 0.55 ± 0.00 | 0.21 ± 0.01 | 223 ± 1 |
| 0.25% CitA | Atmospheric | 68 | 72 | 4.47 | 0.60 ± 0.01 | 0.23 ± 0.01 | 224 ± 1 |
| 0.25% CitA + 0.08% AA | Atmospheric | 68 | 70 | 4.34 | 0.59 ± 0.02 | 0.23 ± 0.00 | 212 ± 5 |
| 0.25% CitA + 0.08% AA | Reduced | 79 | 70 | 4.36 | 0.52 ± 0.05 | 0.21 ± 0.02 | 216 ± 14 |
| 0.5% CitA | Atmospheric | 78 | 76 | 3.79 | 0.73 ± 0.03 | 0.28 ± 0.01 | 214 ± 8 |
| Syrup | Yield (%) | Carbohydrate Composition (Molar %) | Total (g/kg) | Protein (g/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | UA | ||||
| 0.1% CitA | 21 | - | tr | 1 ± 0 | 1 ± 0 | 32 ± 0 | 1 ± 0 | 60 ± 1 | 5 ± 0 | 510 ± 43 | 12 ± 2 |
| 0.25% CitA | 10 | - | tr | 1 ± 0 | 2 ± 0 | 32 ± 2 | 1 ± 0 | 57 ± 1 | 7 ± 0 | 493 ± 28 | 14 ± 2 |
| 0.5% CitA | 3 | - | 1 ± 0 | 2 ± 0 | 2 ± 0 | 30 ± 1 | 3 ± 1 | 53 ± 0 | 10 ± 2 | 502 ± 91 | 23 ± 0 |
| Syrup | Additive | Surface Stickiness (g) | Stringiness (mm) |
|---|---|---|---|
| Yacon (Hualqui) | 0.1% CitA | 7.57 ± 0.25 | 9.54 ± 0.30 |
| 0.25% CitA | 7.01 ± 0.14 | 8.89 ± 0.18 | |
| 0.5% CitA | 9.06 ± 0.39 | 10.36 ± 0.26 | |
| Yacon (Crespo) | 0.1% CitA | 69.21 ± 5.72 | 43.18 ± 3.42 |
| 0.25% CitA | 5.81 ± 0.33 | 7.36 ± 0.11 | |
| 0.5% CitA | 4.70 ± 0.08 | 6.91 ± 0.07 | |
| Yacon (commercial) | 8.59 ± 0.27 | 10.06 ± 0.26 | |
| Agave (commercial) | 7.44 ± 0.08 | 9.14 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, P.A.R.; Antunes, B.L.; Liu, J.; Ferreira, S.S.; Fernandes, F.; Alves, V.D.; Silva, A.; Nunes, C.; Coelho, E.; Coimbra, M.A. Utilization of Yacon Damaged Roots as a Source of FOS-Enriched Sweet-Tasting Syrup. Appl. Sci. 2024, 14, 894. https://doi.org/10.3390/app14020894
Fernandes PAR, Antunes BL, Liu J, Ferreira SS, Fernandes F, Alves VD, Silva A, Nunes C, Coelho E, Coimbra MA. Utilization of Yacon Damaged Roots as a Source of FOS-Enriched Sweet-Tasting Syrup. Applied Sciences. 2024; 14(2):894. https://doi.org/10.3390/app14020894
Chicago/Turabian StyleFernandes, Pedro A. R., Bruna L. Antunes, Jianing Liu, Sónia S. Ferreira, Filipa Fernandes, Vitor D. Alves, Adriana Silva, Cláudia Nunes, Elisabete Coelho, and Manuel A. Coimbra. 2024. "Utilization of Yacon Damaged Roots as a Source of FOS-Enriched Sweet-Tasting Syrup" Applied Sciences 14, no. 2: 894. https://doi.org/10.3390/app14020894