Photocatalytic Testing Protocol for N-Doped TiO2 Nanostructured Particles under Visible Light Irradiation Using the Statistical Taguchi Experimental Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N-Doped TiO2 Nanoparticles
2.2. Characterization
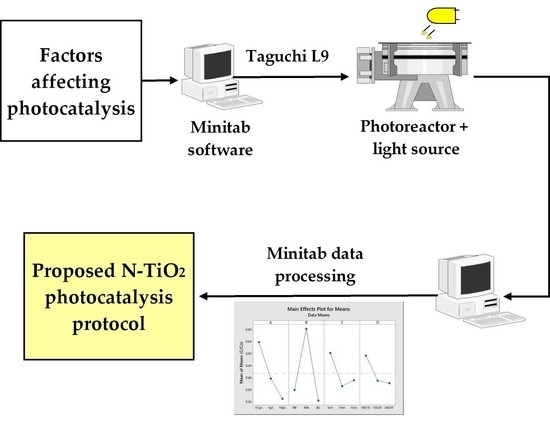
2.3. Experimental Design
2.4. Photocatalytic Test
3. Results and Discussion
3.1. Characterization of the N-TiO2 Powder
3.1.1. XRD Analysis
3.1.2. Raman Analysis
3.1.3. XPS Analysis
3.1.4. UV-Vis Spectrophotometry Analysis
3.1.5. FESEM and TEM Analysis
3.2. Photocatalytic Results
3.3. Taguchi Methods Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, A.A.; Khatun, F.; Monir, M.U.; Ching, S.L. Photocatalyst. In Titanium Dioxide—Advances and Applications; IntechOpen: London, UK, 2021; pp. 1–16. [Google Scholar]
- Wold, A. Photocatalytic Properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjines, R.; Schmid, P.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Bavykin, D.; Friedrich, J.; Walsh, F. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis. Properties and Applications. Adv. Materials. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.; Ansari, M.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. N. J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Kader, S.; Islam, M.; Khan, M. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P.; Kontos, A.; Dunlop, P.; Hamilton, J.; Byrne, J.; O’Shea, K. Review: A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Khaki, M.; Shafeeyan, M.; Raman, A.; Daud, W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Munusamy, S.; Aparna, R.S.L.; Prasad, R.G.S.V. Photocatalytic effect of TiO2 and the effect of dopants on degradation of brilliant green. Sustain. Chem. Process. 2013, 1, 4. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, V. Visible light induced sonophotocatalytic degradation of Reactive Red dye 198 using dye sensitized TiO2. Ultrason. Sonochem. 2007, 14, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng 2019, 36, 749–770. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Arif, M.; Farooqi, Z.; Irfan, A.; Begum, R. Gold nanoparticles and polymer microgels: Last five years of their happy and successful marriage. J. Mol. Liq. 2021, 336, 116270. [Google Scholar] [CrossRef]
- Arif, M. Complete life of cobalt nanoparticles loaded into cross-linked organic polymers: A review. RSC Adv. 2022, 12, 15447–15460. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Anwar, S.; Naeem, H.; Zia, M.; Siddiq, M. Poly(acrylic acid) hydrogel microparticles fabricated with silver nanoparticles: Synthesis, characterization, and catalytic applications. Polym. Eng. Sci. 2020, 60, 2918–2929. [Google Scholar] [CrossRef]
- Javed, M.; Abid, M.; Hussain, S.; Shahwar, D.; Arshad, S.; Ahmad, N.; Arif, M.; Khan, H.; Nadeem, S.; Raza, H.; et al. Synthesis, characterization and photocatalytic applications of s-doped graphitic carbon nitride nanocomposites with nickel doped zinc oxide nanoparticles. Dig. J. Nanomater. Biostruct. 2020, 15, 1097–1105. [Google Scholar]
- Al-hamoud, K.; Shaik, M.; Khan, M.; Alkhathlan, H.Z.; Adil, S.; Kuniyil, M.; Assal, M.; Al-Warthan, A.; Siddiqui, M.H.; Tahir, M.; et al. Pulicaria undulata Extract-Mediated Eco-Friendly Preparation of TiO2 Nanoparticles for Photocatalytic Degradation of Methylene Blue and Methyl Orange. ACS Omega 2022, 7, 4812–4820. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.; Shaik, M.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.H.; Tremel, W.; Tahir, M.N. Advances in Graphene/Inorganic Nanoparticle Composites for Catalytic Applications. Review. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.; Adil, S.; Shaik, M.; Khan, M.; Assal, M.; Siddiqui, M.; Al-Warthan, A. One-Pot Synthesized Pd@N-Doped Graphene: An Efficient Catalyst for Suzuki–Miyaura Couplings. Catalysts 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Adil, S.; Assal, M.; Alharthi, A.; Shaik, M.; Kuniyil, M.; Al-Warthan, A.; Khan, A.; Nawaz, Z.; Shaikh, H.; et al. Solventless Mechanochemical Fabrication of ZnO–MnCO3/N-Doped Graphene Nanocomposite: Efficacious and Recoverable Catalyst for Selective Aerobic Dehydrogenation of Alcohols under Alkali-Free Conditions. Catalysts 2021, 11, 760. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiara, V.; Tayade, R.J. Review: Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G.A. Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.H.; Galante, M.; Martins, T.; Santos, L.; Ely, F.; Longo, C.; Gonçalves, R.; Muniz, S.; Nome, R.A. Real time single TiO2 nanoparticle monitoring of the photodegradation of methylene blue. Sol. Energy 2019, 190, 239–245. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Fallahgar, M.; Pourhasan, F.; Nasiri, M. Taguchi optimization of photodegradation of yellow water of trinitrotoluene production catalyzed by nanoparticles TiO2/N under visible light. Iran. J. Catal. 2017, 7, 317–326. [Google Scholar]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.; Allsopp, D.; Bowen, C.; van Grieken, R.; Marugán, J. Design and validation of a LED-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Naghibi, S.; Sani, M.F.; Hosseini, H.R.M. Application of the statistical Taguchi method to optimize TiO2 nanoparticles synthesis by the hydrothermal assisted sol–gel technique. Ceram. Int. 2014, 40, 4193–4201. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Su, T.-L.; Kung, F.-C.; Wu, T.-J. A study of parameter setting and characterization of visible-light driven nitrogen-modified commercial TiO2 photocatalysts. J. Hazard. Mater. 2011, 190, 938–944. [Google Scholar] [CrossRef]
- Karna, S.K.; Sahai, R. An Overview on Taguchi Method. Int. J. Eng. Math. Sci. 2012, 1, 11–18. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Krishnaiah, K.; Shahabudeen, P. Applied design of experiments and Taguchi methods; PHI Learning Private Limited: New Delhi, India, 2012. [Google Scholar]
- Galata, E.; Georgakopoulou, E.; Kassalia, M.-E.; Papadopoulou-Fermeli, N.; Pavlatou, E.A. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. [Google Scholar] [CrossRef] [Green Version]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Tayade, R.J.; Surolia, P.; Kulkarni, R.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Hanaor, D.; Sorrell, C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Kiwi, J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation ofp-coumaric acid. Appl. Catal. B Environ. 1998, 16, 19–29. [Google Scholar] [CrossRef]
- Liu, G.; Wanga, X.; Chen, Z.; Cheng, H.-M.; Lu, G.Q. The role of crystal phase in determining photocatalytic activity of nitrogen doped TiO2. J. Colloid Interface Sci. 2009, 329, 331–338. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Zhang, X.; Huang, Y.-X.; Li, W.-W.; Yu, H.-Q. Self-induced synthesis of phase-junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature. Nat. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef] [Green Version]
- Patterson, L.A. The Scherrer Formula for I-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Tuschel, D. Raman Spectroscopy and Polymorphism. Spectroscopy 2019, 34, 10–21. [Google Scholar]
- Zhang, W.F.; He, Y.; Zhang, M.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Zaky, A.A.; Christopoulos, E.; Gkini, K.; Arfanis, M.; Sygellou, L.; Kaltzoglou, A.; Stergiou, A.; Tagmatarchis, N.; Balis, N.; Falaras, P. Enhancing efficiency and decreasing photocatalytic degradation of perovskite solar cells using a hydrophobic copper-modified titania electron transport layer. Appl. Catal. B Environ. 2021, 284, 119714. [Google Scholar] [CrossRef]
- Batalovic, K.; Bundaleski, N.; Radakovic, J.; Abazovic, N.; Mitric, M.; Silva, R.; Savic, M.; Belosevic, J.; Rakocevic, Z.; Rangel, C.M. Modification of N-doped TiO2 photocatalysts using noble metals (Pt, Pd)—A combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Myrick, M.L.; Simcock, M.; Baranowski, M.; Brooke, H.; Morgan, S.; McCutcheon, J.N. The Kubelka-Munk Diffuse Reflectance Formula Revisited. Appl. Spectrosc. Rev. 2011, 46, 140–165. [Google Scholar] [CrossRef]
- Lopez, R.; Gomez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An Article on Optics of Paint Layers. Physik 1931, 12, 593–609. [Google Scholar]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.D.; Henley, B.; Sleap, S.; Sloan, S.W. Kinetic model selection and the Hill model in geochemistry. Int. J. Environ. Sci. Technol. 2015, 12, 2545–2558. [Google Scholar] [CrossRef] [Green Version]
- Fei, N.C.; Mehat, N.; Kamar, S. Practical Applications of Taguchi Method for Optimization of Processing Parameters for Plastic Injection Moulding: A Retrospective Review. ISRN Ind. Eng. 2013, 2013, 462174. [Google Scholar] [CrossRef] [Green Version]
- Zalani, N.M.; Kaleji, B.; Mazinani, B. Synthesis and characterisation of the mesoporous ZnO-TiO2 nanocomposite; Taguchi optimisation and photocatalytic methylene blue degradation under visible light. Mater. Technol. 2020, 35, 281–289. [Google Scholar] [CrossRef]
- Manjunatha, C.; Abhishek, B.; Shivaraj, B.; Ashoka, S.; Shashank, M.; Nagaraju, G. Engineering the MxZn1−xO (M = Al3+, Fe3+, Cr3+) nanoparticles for visible light-assisted catalytic mineralization of methylene blue dye using Taguchi design. Chem. Pap. 2020, 74, 2719–2731. [Google Scholar] [CrossRef]
- Giahi, M.; Badalpoor, N.; Habibi, S.; Taghavi, H. Synthesis of CuO/ZnO Nanoparticles and Their Application for Photocatalytic Degradation of Lidocaine HCl by the Trial-and-error and Taguchi Methods. Bull. Korean Chem. Soc. 2013, 34, 2176–2182. [Google Scholar] [CrossRef]
- Sabonian, M.; Mahanpoor, K. Optimization of Photocatalytic Reduction of Cr(VI) in Water with Nano ZnO/Todorokite as a Catalyst: Using Taguchi Experimental Design. Iran. J. Chem. Chem. Eng. 2019, 38, 105–113. [Google Scholar]
- Chandrika, K.C.; Anantharamaiah, P.; Krishna, R.H.; Jineesh, A.; Prabhu, T.N. CoFe2O4-TiO2 a Lethal P-n heterogeneous catalyst for malachite green degradation under visible light and optimisation of parameters by the Taguchi Method. Mater. Technol. 2022, 37, 1877–1889. [Google Scholar] [CrossRef]
- Arimi, A.; Farhadian, M.; Nazar, A.S.; Homayoonfal, M. Assessment of operating parameters for photocatalytic degradation of a textile dye by Fe2O3/TiO2/clinoptilolite nanocatalyst using Taguchi experimental design. Res. Chem. Intermed. 2016, 42, 4021–4040. [Google Scholar] [CrossRef]















| Levels | Parameters (Factors) | |||
|---|---|---|---|---|
| A-Photocatalyst/Pollutant (g/L) | B-Pollutant | C-Distance from Light Source (cm) | D-Irradiation Time (Total min/Measuring Gap Time) | |
| 1 | 0.1 | Methylene Blue | 5 | 100/10 |
| 2 | 1 | Rhodamine B | 10 | 150/30 |
| 3 | 10 | Brilliant Green | 15 | 200/20 |
| Experiment No. | Control Factors | |||
|---|---|---|---|---|
| A | B | C | D | |
| 1 | 0.1 g/L | Methylene Blue | 5 cm | 100 min/10 min |
| 2 | 0.1 g/L | Rhodamine B | 10 cm | 150 min/30 min |
| 3 | 0.1 g/L | Brilliant Green | 15 cm | 200 min/20 min |
| 4 | 1 g/L | Methylene Blue | 10 cm | 200 min/20 min |
| 5 | 1 g/L | Rhodamine B | 15 cm | 100 min/10 min |
| 6 | 1 g/L | Brilliant Green | 5 cm | 150 min/30 min |
| 7 | 10 g/L | Methylene Blue | 15 cm | 150 min/30 min |
| 8 | 10 g/L | Rhodamine B | 5 cm | 200 min/20 min |
| 9 | 10 g/L | Brilliant Green | 10 cm | 100 min/10 min |
| Factor | Effect % |
|---|---|
| A-Photocatalyst/pollutant (g/L) | 28.78 |
| B-Pollutant | 52.21 |
| C-Distance from light source (cm) | 10.95 |
| D-Irradiation time (total min/measuring gap time) | 8.07 |
| Factor | Level for Protocol |
|---|---|
| A-Photocatalyst/pollutant (g/L) | 1 |
| B-Pollutant | Rhodamine B |
| C-Distance from light source (cm) | 10 |
| D-Irradiation time (total min/measuring gap time) | 150/30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassalia, M.-E.; Nikolaou, Z.; Pavlatou, E.A. Photocatalytic Testing Protocol for N-Doped TiO2 Nanostructured Particles under Visible Light Irradiation Using the Statistical Taguchi Experimental Design. Appl. Sci. 2023, 13, 774. https://doi.org/10.3390/app13020774
Kassalia M-E, Nikolaou Z, Pavlatou EA. Photocatalytic Testing Protocol for N-Doped TiO2 Nanostructured Particles under Visible Light Irradiation Using the Statistical Taguchi Experimental Design. Applied Sciences. 2023; 13(2):774. https://doi.org/10.3390/app13020774
Chicago/Turabian StyleKassalia, Maria-Emmanouela, Zoe Nikolaou, and Evangelia A. Pavlatou. 2023. "Photocatalytic Testing Protocol for N-Doped TiO2 Nanostructured Particles under Visible Light Irradiation Using the Statistical Taguchi Experimental Design" Applied Sciences 13, no. 2: 774. https://doi.org/10.3390/app13020774