Pharmacological Properties and Safe Use of 12 Medicinal Plant Species and Their Bioactive Compounds Affecting the Immune System
Abstract
:1. Introduction
2. Materials and Methods
3. Plant Raw Materials and Compounds with Proven Immune-Enhancing Effects
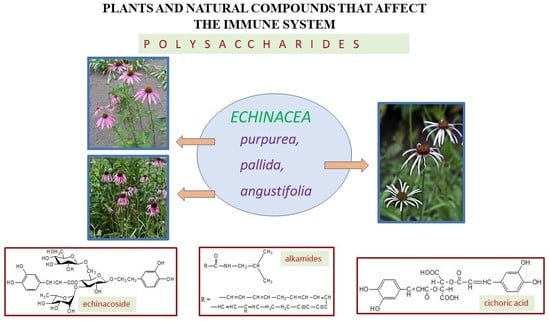
3.1. Echinaceae Purpureae Herba Recens—Echinacea purpurea (L.) Moenh
3.1.1. Pharmacological Properties
3.1.2. Safety of Use
3.2. Echinaceae Purpureae Radix
3.2.1. Pharmacological Properties
3.2.2. Safety of Use
3.3. Echinaceae Pallidae Radix—Echinacea pallida (Nutt.) Nutt.
3.3.1. Pharmacological Properties
3.3.2. Safety of Use
3.4. Echinaceae Angustifoliae Radix—Echinacea angustifolia DC.
3.4.1. Pharmacological Properties
3.4.2. Safety of Use
3.5. Aloe Arboreae Folium—Aloe arborescens Mill.
3.5.1. Pharmacological Properties
3.5.2. Safety of Use
3.6. Panax Quinquefolii Radix—Panax quinquefolius L.
Pharmacological Properties
3.7. Withaniae Radix—Withania somnifera (L.) Dunal
Pharmacological Properties
3.8. Eleutherococci Radix—Eleutherococcus senticosus (Rupr. and Maxim.) Maxim.
3.8.1. Pharmacological Properties
3.8.2. Safety of Use
3.9. Visci Herba—Viscum album L.
Pharmacological Properties
3.10. Uncariae Cortex—Uncaria tomentosa (Willd. Ex Schult.) DC.
3.10.1. Pharmacological Properties
3.10.2. Safety of Use
3.11. Filipendulae Ulmariae Flos et Herba—Filipendula ulmaria (L.) Maxim.
3.11.1. Pharmacological Properties
3.11.2. Safety of Use
3.12. Sambuci Fructus—Sambucus nigra L.
3.12.1. Pharmacological Properties
3.12.2. Safety of Use
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Bratty, M.A.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cock, U.E. A review of the sedative, anti-anxiety and immunostimulant properties of Withania somnifera (L.) Dunal (Ashwagandha). Pharmacogn. Comm. 2023, 13, 15–23. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Cavallo, L.; Mandras, N. Immune defences: A view from the side of the essential oils. Molecules 2023, 28, 435. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, X.; Mizielińska, M.; Ordon, M.; Nawrotek, P. Combinations of Echinacea (Echinacea purpurea) and Rue (Ruta graveolens) plant extracts with lytic phages: A study on Interactions. Appl. Sci. 2023, 13, 4575. [Google Scholar] [CrossRef]
- Koraganji, D.V.; Mounika, A.; Sushanth, P.; Kandra, P. Effect of plant-derived immunomodulators on the immune system. In Nutraceuticals and Functional Foods as Immunomodulators; Springer Nature Singapore: Singapore, 2023; pp. 109–120. [Google Scholar]
- Munro, C. COVID-19: 40% of patients with weakened immune system mount lower response to vaccines. BMJ 2021, 374, 2098. [Google Scholar] [CrossRef] [PubMed]
- Gorji, A.; Ghadiri, M.K. Potential roles of micronutrient deficiency and immune system dysfunction in the coronavirus disease 2019 (COVID-19) pandemic. Nutrition 2021, 82, 111047. [Google Scholar] [CrossRef]
- Suardi, C.; Cazzaniga, E.; Graci, S.; Dongo, D.; Palestini, P. Link between viral infections, immune system, inflammation and diet. Environ. Res. Public Health 2021, 18, 2455. [Google Scholar] [CrossRef]
- Siedlik, J.A.; Benedict, S.H.; Landes, E.J.; Weir, J.P.; Vardiman, J.P.; Gallagher, P.M. Acute bouts of exercise induce a suppressive effect on lymphocyte proliferation in human subjects: A meta-analysis. Brain Behav. Immun. 2016, 56, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Siedlik, J.A.; Deckert, A.J.; Benedict, S.H.; Bhatta, A.; Dunbar, A.J.; Vardiman, J.P.; Gallagher, P.M. T cell activation and proliferation following acute exercise in human subjects is altered by storage conditions and mitogen selection. J. Immunol. Methods 2017, 446, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Alexander, V.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Vollmar, A.M. The role of atrial natriuretic peptide in the immune system. Peptides 2005, 26, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hajto, T.; Hostanska, K.; Frei, K.; Rordorf, C.; Gabius, H.J. Increased secretion of tumour necrosis factor α, interleukin 1, and interleukin 6 by Heimann mononuclear Wells expose to β-galactoside specific lectin from clinically applied mistletoe extract. Canc. Res. 1990, 50, 3322–3226. [Google Scholar]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Rivero Vaccari, J.P.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: Implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Alecu, M.; Geleriu, L.; Coman, G.; Gălăţescu, L. The interleukin-1, interleukin-2, interleukin-6 and tumour necrosis factor alpha serological levels in localised and systemic sclerosis. Rom. J. Intern. Med. 1998, 36, 251–259. [Google Scholar]
- Schulz, V.; Hänsel, R.; Blumenthal, M.; Tyler, V.E. Rational Phytotherapy. A Reference Guide for Physicians and Pharmacists; Springer: Berlin/Heidelberg, Germany, 2004; pp. 381–398. [Google Scholar]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonisation. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef]
- Balzarini, J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007, 5, 583–597. [Google Scholar] [CrossRef]
- Harnett, W.; Harnett, M.M. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim. Biophys. Acta 2001, 1539, 7–15. [Google Scholar] [CrossRef]
- Chirmule, N.; Pahwa, S. Glycoproteins of human immunodeficiency virus type 1: Profound influences on immune functions. Microbiol. Rev. 1996, 60, 386–406. [Google Scholar] [CrossRef]
- Cockfield, S.M. Identifying the patient at risk for the post-transplant lymphoproliferative disorder. Transpl. Infect. Dis. 2001, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Senese, G.; Gallo, C.; Albiani, F.; Romano, L.; d’Ippolito, G.; Manzo, E.; Fontana, A. Antitumor potential of immunomodulatory natural products. Mar. Drugs 2022, 20, 386–413. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Vetvicka, V. β-Glucans, History, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 45–47. [Google Scholar]
- Hajimehdipoor, H.; Khanavi, M.; Shekarchi, M.; Abedi, Z.; Hamedani, M.P. Investigation of the best method for extraction of phenolic compounds from Echinaceae purpurea L. (Moench). J. Med. Plants 2009, 8, 145–152. [Google Scholar]
- Brinkeborn, R.M.; Shah, D.V.; Degenring, F.H. Echinaforce and Rother Echinacea fresh plant preparations in the treatment of a common cold. A randomised, placebo-controlled, double-blind clinical trial. Phytomedicine 1999, 6, 1–6. [Google Scholar] [CrossRef]
- Bräuning, B.; Dorn, B.; Knick, E. Echinaceae purpureae radix zur Stärkung der körpereigenen Abwehr bei grippalen infekten. Z. Phytother. 1992, 13, 7–13. [Google Scholar]
- Senchina, D.S.; Mc Cann, D.A.; Asp, J.M.; Johnson, J.A.; Cunnick, J.E.; Kaiser, M.S.; Kohut, M.L. Changes in immunomodulatory properties of Echinacea spp. root infusions and tinctures stored at 4 °C for four days. Clin. Chim. Acta 2005, 355, 67–82. [Google Scholar] [CrossRef]
- Yadav, P.; El-Kafrawy, S.A.; El-Day, M.M.; Alghafari, W.T.; Faizo, A.A.; Jha, S.K.; Dwivedi, V.D.; Azhar, E.I. Discovery of small molecules from Echinacea angustifolia targeting RNA-dependent RNA polymerase of Japanese Encephalitis virus. Life 2022, 12, 952. [Google Scholar] [CrossRef]
- ESCOP Monographs European Scientific Cooperative On Phytotherapy. Echinaceae angustifoliae Radix. Narrow-Leaved Corneflower Root; Online Series; Thieme: New York, NY, USA, 2019; pp. 1–16. [Google Scholar]
- Bani, S.; Gautam, M.; Sheikh, F.A.; Khan, B.; Satti, N.K.; Suri, K.A.; Qazi, G.N.; Patwardhan, B. Selective Th1 up-regulating the activity of Withania somnifera aqueous extract in an experimental system using flow cytometry. J. Ethnopharmacol. 2006, 107, 107–115. [Google Scholar] [CrossRef]
- Asano, K.; Takahashi, T.; Miyashita, M.; Matsuzaka, A.; Muramatsu, S.; Kuboyama, M.; Kugo, H.; Imai, J. Effect of Eleutherococcus senticosus extract on human physical working capacity. Planta Med. 1986, 52, 175–177. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Nam, K.-H.; Kim, A.; Park, J.; Yoo, E.; Baik, K.; Yu, Y.; Park, M.-H. In vitro and in vivo immunomodulatory effects of syringin. J. Pharmacol. 2001, 53, 1287–1294. [Google Scholar] [CrossRef]
- Reinhard, K.H. Uncaria tomentosa (willd.) D.C.: Cat’s Claw, Uña de Gato, or Savéntaro. J. Altern. Complement. Med. 1999, 5, 143–151. [Google Scholar] [CrossRef]
- Shi, J.S.; Yu, J.X.; Chen, X.P.; Xu, R.X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol. Sin. 2003, 24, 97–101. [Google Scholar] [PubMed]
- Sukhikh, S.; Ivanova, S.; Skrypnik, L.; Bakhtiyarova, A.; Larina, V.; Krol, O.; Prosekov, A.; Frolov, A.; Povydysh, M.; Babich, O. Study of the antioxidant properties of Filipendula ulmaria and Alnus glutinosa. Plants 2022, 16, 2415. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. 2022, 15, 100437. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 665. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef]
- Blumenthal, M.; Goldberg, A.; Brinckann, J. Herbal Medicine. Expanded Commission E Monographs. Echinacea purpurea Herb, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 96–97. [Google Scholar]
- Hoheisel, O.; Sandberg, M.; Bertram, S.; Bulitta, M.; Schäfer, M. Echinagard treatment shortens the course of the common cold: A double-blind, placebo-controlled clinical trial. Eur. J. Clin. Res. 1997, 9, 261–268. [Google Scholar]
- Nowak, G. Plant raw materials and natural substances influencing the immune system. Herba Pol. 2010, 56, 79–91. [Google Scholar]
- Pilarska, G.; Twarużek, M.; Ałtyn, I. The presence of molds and their secondary metabolites in purple coneflower-based dietary supplements (Echinacea purpurea (L.) Moench). Toxins 2022, 14, 607. [Google Scholar] [CrossRef]
- Linde, K.; Barret, B.; Bauer, R.; Melchart, D.; Woelkart, K. Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2006, 1, CD000530. [Google Scholar]
- EMA European Medicines Agency. Echinacea purpurea (L.) Moench. Herba recens; Thieme: New York, NY, USA; London, UK, 2015. [Google Scholar]
- ESCOP Monographs European Scientific Cooperative On Phytotherapy. Echinaceae purpureae Herba, 2nd ed.; Supplement; Thieme: New York, NY, USA, 2009; pp. 91–101. [Google Scholar]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.H.; Schoop, R.; Gertsch, J. Synergistic immunomopharmacological effects of N-alkylamines in Echinacea purpurea herbal and root extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, M.; Kim, D.; Dong, H.O.; Prasad, K.S.; Eun, S.; Lee, J. Echinacea purpurea extract enhances natural killer cell activity in vivo by upregulating MHC II and Th1-type CD4+ T cell responses. J. Med. Food 2021, 24, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Caruso, T.J.; Gwaltney, J.M. Treatment of the common cold with Echinacea a structured review. Clin. Infect. Dis. 2005, 40, 807–810. [Google Scholar] [CrossRef] [PubMed]
- EMA European Medicines Agency. Echinacea purpurea (L.) Moench; Radix: London, UK, 2017. [Google Scholar]
- ESCOP Monographs European Scientific Cooperative On Phytotherapy. Echinaceae purpureae Radix, 2nd ed.; Supplement; Thieme: New York, NY, USA, 2009; pp. 102–109. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckann, J. Herbal Medicine. Expanded Commission E Monographs. Echinacea pallida Herb, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 93–95. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 927–954. [Google Scholar]
- EMA European Medicines Agency. Echinacea pallidae (Nutt.) Nutt; Radix: London, UK, 2018. [Google Scholar]
- ESCOP Monographs European Scientific Cooperative On Phytotherapy. Echinaceae pallidae Radix, 2nd ed.; Supplement; Thieme: New York, NY, USA, 2009; pp. 87–90. [Google Scholar]
- Melchart, D.; Linde, K.; Worku, F.; Sarkady, L.; Holzmann, M.; Jurcic, K.; Wagner, H. Results of five randomized studies on the immunostimulatory activity of preparations of Echinacea. Altern. Complement. Med. 1995, 1, 145–160. [Google Scholar] [CrossRef]
- Tragni, E.; Galli, C.I.; Tubaro, A.; Del Negro, P.; Della Loggia, R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmacol. Res. Commun. 1988, 5, 87–90. [Google Scholar] [CrossRef]
- Turner, R.B.; Bauer, R.; Woelkart, K.; Hulsey, T.C.; Gangemi, J.D. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N. Engl. J. Med. 2005, 353, 341–348. [Google Scholar] [CrossRef]
- EMA European Medicines Agency. Echinacea angustifolia DC; Radix: London, UK, 2012. [Google Scholar]
- Yagi, A.; Makino, K.; Nishioka, I.; Kuchino, Y. Aloe mannan, polysaccharide, from Aloe arborescens var. natalensis. Planta Med. 1977, 31, 17–20. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Mellick, P.W.; Olson, G.R.; Felton, R.P.; Thorn, B.T.; Beland, F.A. Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis Miller (Aloe vera) in F344/N rats. Toxicol. Sci. 2013, 131, 26–39. [Google Scholar] [CrossRef]
- Yagi, A.; Nishimura, H.; Shida, T.; Nishioka, I. Structure determination of polysaccharides in Aloe arborescens var. natalensis. Planta Med. 1986, 52, 213–218. [Google Scholar] [CrossRef]
- Assinewe, V.A.; Amason, J.T.; Aubry, A.; Mullin, J.; Lemaire, I. Extractable polysaccharides of Panax quinquefolius L. (North American ginseng) root stimulate TNF-a production by alveolar macrophages. Phytomedicine 2002, 9, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, H.R.; Sham, J.; Chau, L.A.; Madrenas, J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: Characterisation of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 2012, 142, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Azike, C.G.; Charpentier, P.A.; Lui, E.M.K. Stimulation and suppression of innate immune function by American ginseng polysaccharides: Biological relevance and identification of bioactives. Pharm. Res. 2015, 32, 876–897. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.; Singh, J.; Khajuria, A.; Suri, K.A.; Satti, N.K.; Singh, S.; Kaul, M.K.; Kumar, A.; Bhatia, A.; Qazi, G.N. A standardised root extract of Withania somnifera and its major constituent withanolide—A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarisation in BALB/c mice. Life Sci. 2007, 80, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. & Maxim) Maxim (Araliaceae) as an adaptogen: A closer look. J. Etnopharmacol. 2000, 72, 345–393. [Google Scholar]
- Liu, K.Y.; Wu, Y.-C.; Liu, I.-M.; Yu, W.C.; Cheng, J.-T. Release of acetylocholine by syringin, an active principle of Eleutherococcus senticosus, to raise insulin secretion in Wistar rats. Neurosci. Lett. 2008, 434, 195–199. [Google Scholar] [CrossRef]
- EMA European Medicines Agency. Evaluation of Medicines for Human Use. In Eleutherococcus senticosus (Rupr. et Maxim) Maxim; EMA European Medicines Agency: London, UK, 2014; Available online: https//www.ema.europa.eu/en (accessed on 23 May 2023).
- Kleszken, E.; Timar, A.; Memete, A.; Miere, F.; Vicas, S.I. On overview of bioactive compounds, biological and pharmacological effects of mistletoe (Viscum album L.). Pharmacophore 2022, 13, 10–26. [Google Scholar] [CrossRef]
- Wagner, H.K.M. Immunostimmulants and adaptogens from plants. In Phytochemistry of Medicinal Plants; Arnanson, J.T., Mata, R., Romeo, J.T., Eds.; Plenum Press: New York, NY, USA; London, UK, 1994; pp. 1–18. [Google Scholar]
- Kutan, R.; Kutan, G. Immunomodulatory activity of a peptide isolated from Viscum album extract (NSC 635 089). Immunol. Investig. 1992, 21, 285–296. [Google Scholar] [CrossRef]
- Braedel-Ruoff, S. Immunomodulatory effects of Viscum album extracts on natural killer cells: Review of clinical trials. Forsch. Komplementmed. 2010, 17, 63–73. [Google Scholar] [CrossRef]
- Hajtó, T.; Fodor, K.; Perjési, P.; Németh, P. Difficulties and perspectives of immunomodulatory therapy with mistletoe lectins and standardized mistletoe extracts in evidence-based medicine. Evid. Based Complement. Alternat. Med. 2009, 25, 298972. [Google Scholar]
- Dioguardi, M.; Spirito, F.; Sovereto, D.; Ballini, A.; Alovisi, M.; Muzio, L.L. Application of the extracts of Uncaria tomentosa in endodontics and oral medicine: Scoping review. J. Clin. Med. 2022, 11, 5024. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, K.; Laus, G.; Wurm, M.; Dierich, M.P.; Teppner, H. Uncaria tomentosa (Willd.) DC.-ethnomedicinal use and new pharmacological, toxicological and botanical results. J. Ethnopharmacol. 1998, 64, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Castihos, L.G.; Rezer, J.F.P.; Ruchel, J.B.; Thorstenberg, M.L.; Jaques, J.A.S.; Schlemmer, J.B.; Doleski, P.H.; Rossato, M.F.; da Silva, M.A.; Cassali, M.A.; et al. Effect of Uncaria tomentosa extract on purinergic enzyme activities in lymphocytes of rats submitted to experimental adjuvant arthritis model. BMC Complement. Altern. Med. 2015, 15, 189–198. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cortex Uncariae. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3, pp. 349–358. [Google Scholar]
- ESCOP Monographs. European Scientific Cooperative on Phytotherapy. Uncariae tomentose Cortex. In Cat’s Claw Bark; Online Series; 2018; pp. 1–14. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine. Expanded Commission E Monographs. Meadowsweet, 1st ed.; American Botanical Council: Austin, TX, USA, 2000; pp. 253–256. [Google Scholar]
- ESCOP Monographs. European Scientific Cooperative on Phytotherapy. In Filipendulae ulmariae Herba, 2nd ed.; Thieme: New York, NY, USA, 2003; pp. 157–161. [Google Scholar]
- EMA European Medicines Agency. Filipendula ulmaria L. Maxim. Herba; EMA. European Medicines Agency: Amsterdam, The Netherlands; London, UK, 2011. [Google Scholar]
- Michel, P.; Granica, S.; Rosińska, K.; Glige, M.; Rojek, J.; Poraj, Ł.; Olszewska, A.M. The effect of standardised leaf extracts of Gaultheria procumbens on multiple oxidants, inflammation-related enzymes, and pro-oxidant and pro-inflammatory functions of human neutrophils. Molecules 2022, 27, 3357. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Sambucus nigra (black elder) as alternative treatment for cold and flu. Nat. Public Health Emerg. Collect. 2021, 21, 405–414. [Google Scholar] [CrossRef]
- EMA. European Medicines Agency. Sambucus nigra L. Radix; EMA. European Medicines Agency: Amsterdam, The Netherlands; London, UK, 2014. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geszke-Moritz, M.; Nowak, G.; Moritz, M. Pharmacological Properties and Safe Use of 12 Medicinal Plant Species and Their Bioactive Compounds Affecting the Immune System. Appl. Sci. 2023, 13, 6477. https://doi.org/10.3390/app13116477
Geszke-Moritz M, Nowak G, Moritz M. Pharmacological Properties and Safe Use of 12 Medicinal Plant Species and Their Bioactive Compounds Affecting the Immune System. Applied Sciences. 2023; 13(11):6477. https://doi.org/10.3390/app13116477
Chicago/Turabian StyleGeszke-Moritz, Małgorzata, Gerard Nowak, and Michał Moritz. 2023. "Pharmacological Properties and Safe Use of 12 Medicinal Plant Species and Their Bioactive Compounds Affecting the Immune System" Applied Sciences 13, no. 11: 6477. https://doi.org/10.3390/app13116477