Acetylated Trifluoromethyl Diboronic Acid Anthracene with a Large Stokes Shift and Long Excitation Wavelength as a Glucose-Selective Probe
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Measurement of Saccharide-Dependent Fluorescence
2.2.2. Measurement of Decay Time
2.2.3. Relative Quantum Yield
3. Results
3.1. Synthesis
3.1.1. 4,4,5,5-Tetramethyl-2-(2-methyl-4-(trifluoromethyl)phenyl)-1,3,2-dioxoborolane (Compound 1)
3.1.2. 2-(2-(Bromomethyl)-4-(trifluoromethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxoborolane (Compound 2)
3.1.3. 2-Acetyl-9,10-dimethylanthracene (Compound 3)
3.1.4. 2-Acetyl-9,10-bis(bromomethyl)anthracene (Compound 4)
3.1.5. Tert-butyl 3-(([3-acetyl-10-(([3-methacrylamidopropyl]amino)methyl)anthracen-9-yl]methyl)amino)propanoate (Compound 5)
3.1.6. (2-([((3-Acetyl-10-[((2-borono-5-[trifluoromethyl]benzyl)[3-methacrylamidopropyl]amino)methyl)anthracen-9-yl]methyl)(3-[tert-butoxy]-3-oxopropyl)amino)methyl]-4-(trifluoromethyl)phenyl)boronic Acid (Compound 6)
3.1.7. 3-(((3-Acetyl-10-(((2-borono-5-(trifluoromethyl)benzyl)(3-methacrylamidopropyl)amino)methyl)anthracen-9-yl)methyl)(2-borono-5-(trifluoromethyl)benzyl)amino)propanoic Acid (Compound 7a)
3.1.8. 3-((2-Borono-5-(trifluoromethyl)benzyl)((10-(((2-borono-5-(trifluoromethyl)benzyl)(3-methacrylamidopropyl)amino)methyl)anthracen-9-yl)methyl)amino)propanoic acid (Compound 7b)
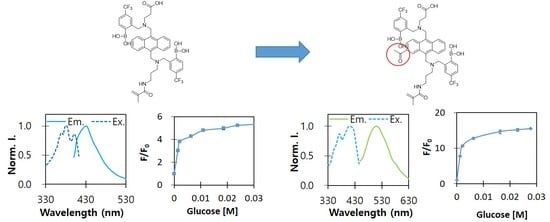
3.2. Fluorogenic Optical Properties
3.3. Measurement of Oxidation Resistance
3.4. Glucose-Dependent Optical Characterisitcs
3.5. Interference-Dependent Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Koya, D.; King, G.L. Protein kinase C activation and the development of diabetic complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Marcantonio, E.R.; Mangione, C.M.; Thomas, E.J.; Polanczyk, C.A.; Cook, E.F.; Sugarbaker, D.J.; Donaldson, M.C.; Poss, R.; Ho, K.L.L.; et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999, 100, 1043–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2007, 34, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Farmer, A.; Wade, A.; Goyder, E.; Yudkin, P.; French, D.; Craven, A.; Holman, R.; Kinmonth, A.-L.; Neil, A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: Open parallel group randomised trial. BMJ 2007, 335, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, B.H. The current environment of CGM technologies. J. Diabetes Sci. Technol. 2007, 1, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Funtanilla, V.D.; Candidate, P.; Caliendo, T.; Hilas, O. Continuous glucose monitoring: A review of available systems. Pharm. Ther. 2019, 44, 550–553. [Google Scholar]
- Petrie, J.R.; Peters, A.L.; Bergenstal, R.M.; Holl, R.W.; Fleming, G.A.; Heinemann, L. Improving the clinical value and utility of CGM systems: Issues and recommendations. Diabetologia 2017, 60, 2319–2328. [Google Scholar] [CrossRef] [Green Version]
- Mano, N. Engineering glucose oxidase for bioelectrochemical applications. Bioelectrochemistry 2019, 128, 218–240. [Google Scholar] [CrossRef]
- Beaufils, C.; Man, H.; Poulpiquet, A.; Mazurenko, I.; Lojou, E. From enzyme stability to enzymatic bioelectrode stabilization processes. Catalysts 2021, 11, 497. [Google Scholar] [CrossRef]
- Joseph, J.I. Review of the long-term implantable senseonics continuous glucose monitoring system and other continuous glucose monitoring systems. J. Diabetes Sci. Technol. 2021, 15, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Colvin, A.E.; Jiang, H. Increased in vivo stability and functional lifetime of an implantable glucose sensor through platinum catalysis. J. Biomed. Mater. Res. Part A 2013, 101, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- James, T.D.; Samankumara Sandanayake, K.R.A.; Iguchi, R.; Shinkai, S. Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar] [CrossRef]
- Heo, Y.J.; Shibata, H.; Okitsu, T.; Kawanishi, T.; Takeuchi, S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc. Natl. Acad. Sci. USA 2011, 108, 13399–13403. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Wang, H.; Bian, Z.; Sun, J.; Liu, A.; Fang, H.; Liu, B.; Yao, Q.; Wu, Z. Recent development of boronic acid-based fluorescent sensors. RSC Adv. 2018, 8, 29400–29427. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Chou, K.H.; Queenan, B.N.; Pennathur, S.; Bazan, G.C. Molecular design of a new diboronic acid for the electrohydrodynamic monitoring of glucose. Angew. Chem. Int. Ed. Engl. 2019, 58, 10612–10615. [Google Scholar] [CrossRef]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.A.; Barbu, M.; Halim, M.; Pallavi, P.; Kim, B.; Kolpashchikov, D.M.; Pecic, S.; Taylor, S.; Worgall, T.S.; Stojanovic, M.N. Recognition and sensing of low-epitope targets via ternary complexes with oligonucleotides and synthetic receptors. Nat. Chem. 2014, 6, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Colvin, A.E., Jr.; Mortellaro, M.A.; Modzelewska, A. Oxidation Resistance Indicator Molecules. U.S. Patent No. 7,851,225 B2, March 2010. [Google Scholar]
- DeHennis, A.; Getzlaff, S.; Grice, D.; Mailand, M. An NFC-Enabled CMOS IC for a wireless fully implantable glucose Sensor. IEEE J. Biomed. Health Inform. 2015, 20, 18–28. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Di Costanzo, L.; Bakayoko, G.; Panunzi, B. A novel DR/NIR T-shaped AIEgen: Synthesis and X-ray crystal structure study. Crystals 2020, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, T.; Romey, M.A.; Zhu, P.C.; Holody, M.Z.; Shinkai, S. A study of boronic acid based fluorescent glucose sensors. J. Fluoresc. 2004, 14, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, R.; Yue, X.; Zhou, Z.; Bai, L.; Tong, Y.; Wang, B.; Gu, D.; Wang, S.; Qiao, Y.; et al. Synthesis of diboronic acid-based fluorescent probes for the sensitive detection of glucose in aqueous media and biological matrices. ACS Sens. 2021, 6, 1543–1551. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Wei, Y. A sandwich boronate affinity sorbent assay for glucose detection facilitated by boronic acid-terminated fluorescent polymers. Sens. Actuators B Chem. 2017, 247, 595–601. [Google Scholar] [CrossRef]
- Rastede, E.E.; Tanha, M.; Yaron, D.; Watkins, S.C.; Waggoner, A.S.; Armitage, B.A. Spectral fine tuning of cyanine dyes: Electron donor—acceptor substituted analogues of thiazole orange. Photochem. Photobiol. Sci. 2015, 14, 1703–1712. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Sutherland, K.; Mahoney, J.R.; Coury, A.J.; Eaton, J.W. Degradation of biomaterials by phagocyte-derived oxidants. J. Clin. Investig. 1993, 92, 2360–2367. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.D.; Frimat, K.A.; Fyles, T.M.; Flower, S.E.; James, T.D. Boronic acid based photoinduced electron transfer (PET) fluorescence sensors for Saccharides. New J. Chem. 2010, 34, 2922–2931. [Google Scholar] [CrossRef]
- Zwinkels, J.C.; DeRose, P.C.; Leland, J.E. Chapter 7—Spectral Fluorescence Measurements. In Experimental Methods in the Physical Sciences; Germer, T.A., Zwinkels, J.C., Tsai, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 46, pp. 221–290. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Song, I.; Park, C.S.; Yim, H.-s.; Kim, J.H. Acetylated Trifluoromethyl Diboronic Acid Anthracene with a Large Stokes Shift and Long Excitation Wavelength as a Glucose-Selective Probe. Appl. Sci. 2022, 12, 2782. https://doi.org/10.3390/app12062782
Choi H, Song I, Park CS, Yim H-s, Kim JH. Acetylated Trifluoromethyl Diboronic Acid Anthracene with a Large Stokes Shift and Long Excitation Wavelength as a Glucose-Selective Probe. Applied Sciences. 2022; 12(6):2782. https://doi.org/10.3390/app12062782
Chicago/Turabian StyleChoi, Hongsik, Inhyeok Song, Chul Soon Park, Heung-seop Yim, and Joong Hyun Kim. 2022. "Acetylated Trifluoromethyl Diboronic Acid Anthracene with a Large Stokes Shift and Long Excitation Wavelength as a Glucose-Selective Probe" Applied Sciences 12, no. 6: 2782. https://doi.org/10.3390/app12062782