New Design of a Sample Cell for Neutron Reflectometry in Liquid–Liquid Systems and Its Application for Studying Structures at Air–Liquid and Liquid–Liquid Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Cell Design
2.3. Neutron Transmittance Measurements
2.4. Neutron Reflectometry Measurements
3. Results
3.1. Neutron Transmittance of the Sample Cell and Toluene-d8 Solution
3.2. Measurements of Neutron Reflection from Air–Liquid Interfaces
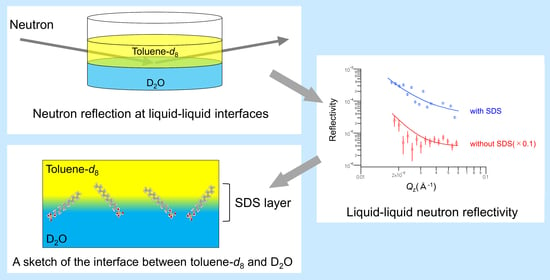
3.3. Measurements of Neutron Reflection from Liquid–Liquid Interfaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scoppola, E.; Watkins, E.B.; Campbell, R.A.; Konovalov, O.; Girard, L.; DufrÞche, J.-F.; Ferru, G.; Fragneto, G.; Diat, O. Solvent Extraction: Structure of the Liquid–Liquid Interface Containing a Diamide Ligand. Angew. Chem. Int. Ed. 2016, 55, 9326–9330. [Google Scholar] [CrossRef]
- Zarbakhsh, A.; Querol, A.; Bowers, J.; Yaseen, M.; Lu, J.R.; Webster, J.R.P. Neutron Reflection from the Liquid–Liquid Interface: Adsorption of Hexadecylphosphorylcholine to the Hexadecane–Aqueous Solution Interface. Langmuir 2005, 21, 11704–11709. [Google Scholar] [CrossRef]
- Nishi, N.; Uruga, T.; Tanida, H. Potential dependent structure of an ionic liquid at ionic liquid j water interface probed by X-ray reflectivity measurements. J. Electroanal. Chem. 2015, 759, 129–136. [Google Scholar] [CrossRef]
- Schlossman, M.L. Liquid–liquid interfaces: Studied by X-ray and neutron scattering. Curr. Opin. Colloid Interface Sci. 2002, 7, 235–243. [Google Scholar] [CrossRef]
- Tanida, H.; Nagatani, H.; Harada, M. Development of the total-reflection XAFS method for the liquid–liquid interface. J. Phys. Conf. Ser. 2007, 83, 012019. [Google Scholar]
- Tummino, A.; Scoppola, E.; Fragneto, G.; Gutfreund, P.; Maestro, A.; Dryfe, R.A.W. Neutron reflectometry study of the interface between two immiscible electrolyte solutions: Effects of electrolyte concentration, applied electric field, and lipid adsorption. Electrochim. Acta 2021, 384, 138336. [Google Scholar] [CrossRef]
- Scoppola, E.; Micciulla, S.; Kuhrts, L.; Maestro, A.; Campbell, R.A.; Konovalov, O.V.; Fragneto, G.; Schneck, E. Reflectometry Reveals Accumulation of Surfactant Impurities at Bare Oil/Water Interfaces. Molecules 2019, 24, 4113. [Google Scholar]
- Darwish, T.A.; Luks, E.; Moraes, G.; Yepuri, N.R.; Holden, P.J.; James, M. Synthesis of deuterated [D32]oleic acid and its phospholipid derivative [D64]dioleoyl-sn-glycero-3-phosphocholine. J. Label Compd. Radiopharm. 2013, 56, 520–529. [Google Scholar] [CrossRef]
- Akutsu-Suyama, K.; Cagnes, M.; Tamura, K.; Kanaya, T.; Darwish, T.A. Controlled deuterium labelling of imidazolium ionic liquids to probe the fine structure of the electrical double layer using neutron reflectometry. Phys. Chem. Chem. Phys. 2019, 21, 17512–17516. [Google Scholar] [CrossRef]
- Sun, H.; Zielinska, K.; Resmini, M.; Zarbakhsh, A. Interactions of NIPAM nanogels with model lipid multi-bilayers: A neutron reflectivity study. J. Colloid Interface Sci. 2019, 536, 598–608. [Google Scholar] [CrossRef]
- Akutsu, K.; Cagnes, M.; Niizeki, T.; Hasegawa, Y.; Darwish, T.A. Penetration behavior of an ionic liquid in thin-layer silica coating: Ionic liquid deuteration and neutron reflectivity analysis. Physical B 2018, 551, 262–265. [Google Scholar] [CrossRef]
- Penfold, J.; Tucker, I.; Thomas, R.K.; Zhang, J. Adsorption of Polyelectrolyte/Surfactant Mixtures at the Air—Solution Interface: Poly(ethyleneimine)/Sodium Dodecyl Sulfate. Langmuir 2005, 21, 10061–10073. [Google Scholar] [CrossRef]
- Li, N.; Thomas, R.K.; Rennie, A.R. Effect of pH, surface charge and counter-ions on the adsorption of sodium dodecyl sulfate to the sapphire/solution interface. J. Colloid Interface Sci. 2012, 378, 152–158. [Google Scholar] [CrossRef] [Green Version]
- de Aguiar, H.B.; Strader, M.L.; de Beer, A.G.F.; Roke, S. Surface Structure of Sodium Dodecyl Sulfate Surfactant and Oil at the Oil-in-Water Droplet Liquid/Liquid Interface: A Manifestation of a Nonequilibrium Surface State. J. Phys. Chem. B 2011, 115, 2970–2978. [Google Scholar] [CrossRef]
- Conboy, J.C.; Messmer, M.C.; Richmond, G.L. Investigation of Surfactant Conformation and Order at the Liquid-Liquid Interface by Total Internal Reflection Sum-Frequency Vibrational Spectroscopy. J. Phys. Chem. 1996, 100, 7617–7622. [Google Scholar] [CrossRef]
- Schweighofer, K.J.; Essmann, U.; Berkowitz, M. Simulation of Sodium Dodecyl Sulfate at the Water-Vapor and Water-Carbon Tetrachloride Interfaces at Low Surface Coverage. J. Phys. Chem. B 1997, 101, 3793–3799. [Google Scholar] [CrossRef]
- Takeda, M.; Arai, M.; Suzuki, J.; Yamazaki, D.; Soyama, K.; Maruyama, R.; Hayashida, H.; Asaoka, H.; Yamazaki, T.; Kubota, M.; et al. Current Status of a New Polarized Neutron Reflectometer at the Intense Pulsed Neutron Source of the Materials and Life Science Experimental Facility (MLF) of J-PARC. Chin. J. Phys. 2012, 50, 161–170. [Google Scholar]
- Akutsu-Suyama, K. Fine-Structure Analysis of Perhydropolysilazane-Derived Nano Layers in Deep-Buried Condition Using Polarized Neutron Reflectometry. Polymers 2020, 12, 2180. [Google Scholar] [CrossRef]
- Sakasai, K.; Satoh, S.; Seya, T.; Nakamura, T.; Toh, K.; Yamagishi, H.; Soyama, K.; Yamazaki, D.; Maruyama, R.; Oku, T.; et al. Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex III: Neutron Devices and Computational and Sample Environments. Quantum Beam Sci. 2017, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Yamada, N.L.; Torikai, N.; Mitamura, K.; Sagehashi, H.; Sato, S.; Seto, H.; Sugita, T.; Goto, S.; Furusaka, M.; Oda, T.; et al. Design and performance of horizontal-type neutron reflectometer SOFIA at J-PARC/MLF. Eur. Phys. J. Plus 2011, 126, 108. [Google Scholar] [CrossRef]
- Mitamura, K.; Yamada, N.L.; Sagehashi, H.; Torikai, N.; Arita, H.; Terada, M.; Kobayashi, M.; Sato, S.; Seto, H.; Goko, S.; et al. Novel neutron reflectometer SOFIA at J-PARC/MLF for in-situ soft-interface characterization. Polym. J. 2013, 45, 100–108. [Google Scholar] [CrossRef]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Purcell, I.P.; Lu, J.R.; Thomas, R.K.; Howe, A.M.; Penfold, J. Adsorption of Sodium Dodecyl Sulfate at the Surface of Aqueous Solutions of Poly(vinylpyrrolidone) Studied by Neutron Reflection. Langmuir 1998, 14, 1637–1645. [Google Scholar] [CrossRef]
- Ocko, B.M.; Wu, X.Z.; Sirota, E.B.; Sinha, S.K.; Deutsch, M. X-ray Reflectivity Study of Thermal Capillary Waves on Liquid Surfaces. Phys. Rev. Lett. 1994, 72, 242–245. [Google Scholar] [CrossRef]
- Bowers, J.; Zarbakhsh, A.; Webster, J.R.P.; Hutchings, L.R.; Richards, R.W. Neutron Reflectivity Studies at Liquid–Liquid Interfaces: Methodology and Analysis. Langmuir 2001, 17, 140–145. [Google Scholar] [CrossRef]
- Bartell, L.S.; Kuchitsu, K.; deNeui, R.J. Mean and Equilibrium Molecular Structures of Methane and Deuteromethane as Determined by Electron Diffraction. J. Chem. Phys. 1961, 35, 1211–1218. [Google Scholar] [CrossRef]
- Bartell, L.S.; Roskos, R.R. Isotope Effects on Molar Volume and Surface Tension: Simple Theoretical Model and Experimental Data for Hydrocarbons. J. Chem. Phys. 1966, 44, 457–463. [Google Scholar] [CrossRef]
- Wade, D. Deuterium isotope effects on noncovalent interactions between molecules. Chem.-Biol. Interact. 1999, 117, 191–217. [Google Scholar] [CrossRef]
- Dunitz, J.; Ibberson, R.M. Is Deuterium Always Smaller than Protium? Angew. Chem. Int. Ed. 2008, 47, 4208–4210. [Google Scholar] [CrossRef]
- Rolle, M.; Jin, B. Normal and Inverse Diffusive Isotope Fractionation of Deuterated Toluene and Benzene in Aqueous Systems. Environ. Sci. Technol. Lett. 2017, 4, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.A.; Arteta, M.Y.; Angus-Smyth, A.; Nylander, T.; Varga, I. Effects of Bulk Colloidal Stability on Adsorption Layers of Poly(diallyldimethylammonium Chloride)/Sodium Dodecyl Sulfate at the Air-Water Interface Studied by Neutron Reflectometry. J. Phys. Chem. B 2011, 115, 15202–15213. [Google Scholar] [CrossRef]
- Braun, L.; Uhlig, M.; von Klitzing, R.; Campbell, R.A. Polymers and surfactants at fluid interfaces studied with specular neutron reflectometry. Adv. Colloid Interface Sci. 2017, 247, 130–148. [Google Scholar] [CrossRef]
- Campbell, R.A. Recent advances in resolving kinetic and dynamic processes at the air/water interface using specular neutron reflectometry. Curr. Opin. Colloid Interface Sci. 2018, 37, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Pannetier, M.; Doan, T.D.; Ott, F.; Berger, S.; Persat, N.; Fermon, C. Polarised neutron reflectometry for GMR sensors optimization. Europhys. Lett. 2003, 64, 524–528. [Google Scholar]
- Colombi, P.; Agnihotri, D.K.; Asadchikov, V.E.; Bontempi, E.; Bowen, D.K.; Chang, C.H.; Depero, L.E.; Farnworth, M.; Fujimoto, T.; Gibaud, A.; et al. Reproducibility in X-ray reflectometry: Results from the first world-wide round-robin experiment. J. Appl. Cryst. 2008, 41, 143–152. [Google Scholar] [CrossRef]
- Gerelli, Y. Aurore: New software for neutron reflectivity data analysis. J. Appl. Cryst. 2016, 49, 330–339. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akutsu-Suyama, K.; Yamada, N.L.; Ueda, Y.; Motokawa, R.; Narita, H. New Design of a Sample Cell for Neutron Reflectometry in Liquid–Liquid Systems and Its Application for Studying Structures at Air–Liquid and Liquid–Liquid Interfaces. Appl. Sci. 2022, 12, 1215. https://doi.org/10.3390/app12031215
Akutsu-Suyama K, Yamada NL, Ueda Y, Motokawa R, Narita H. New Design of a Sample Cell for Neutron Reflectometry in Liquid–Liquid Systems and Its Application for Studying Structures at Air–Liquid and Liquid–Liquid Interfaces. Applied Sciences. 2022; 12(3):1215. https://doi.org/10.3390/app12031215
Chicago/Turabian StyleAkutsu-Suyama, Kazuhiro, Norifumi L. Yamada, Yuki Ueda, Ryuhei Motokawa, and Hirokazu Narita. 2022. "New Design of a Sample Cell for Neutron Reflectometry in Liquid–Liquid Systems and Its Application for Studying Structures at Air–Liquid and Liquid–Liquid Interfaces" Applied Sciences 12, no. 3: 1215. https://doi.org/10.3390/app12031215