The Influence of Processing Time on Morphology, Structure and Functional Properties of PEO Coatings on AZ63 Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and PEO Experimental Parameters
2.2. Characterization Methods
3. Results and Discussions
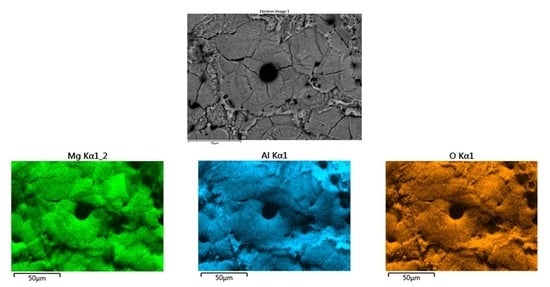
3.1. SEM-EDS Sample Surface Analysis
3.1.1. Morphology and Topography
- –
- standard deviation of the height distribution, or RMS surface roughness
- –
- mean surface roughness
3.1.2. Surface Elemental Composition
3.2. Cross-Section SEM-EDS Analysis
3.2.1. Cross-Section Morphology and Elemental Composition
3.2.2. Coating Thickness
3.3. X-Ray Diffraction Analysis
- -
- Decomposition of the electrolyte under the influence of the strong discharges that penetrate the layer, which leads to the presence of electrolyte species on the surface of the pancakes and at the coating/substrate interface after a single discharge [5]. Thus, aluminum ions from the electrolyte and oxygen species present in the pores and discharge channel forms aluminum oxide allotropic forms;
- -
- Ejecting process of molten magnesium and alloying elements (approx. 6% aluminum), followed by an oxidation process in the discharge channel, and finally quenching and deposition. The formation of γ-alumina is associated with a fast cooling rate [43].
3.4. Vickers Micro-Hardness
3.5. Potentiodynamic Polarization Tests
- -
- Coating thickness, crystalline phase composition, roughness, level of porosity and defects for mechanical properties;
- -
- Coating thickness, structure and chemical composition, level of porosity and the integrity of the coating/substrate barrier layer for anti-corrosion properties.
4. Conclusions
- -
- The PEO coatings formed on AZ63 magnesium alloy, in NaAlO2 electrolyte without any additives, were mainly composed of Mg, Al and O;
- -
- Although the PEO coatings have similar characteristic surface structures, a decrease in relative apparent porosity and an increase in surfaces roughness with increasing processing time was observed;
- -
- The PEO coatings were mainly composed of the MgAl2O4 crystalline phase, with its relative ratio increasing with processing time;
- -
- The coatings (and barrier layer) thickness and compactness increased with processing time.
- -
- A 2 order of magnitude improvement of the corrosion protective properties was correlated with an increase in MgAl2O4 content, coatings thickness and a decrease in apparent porosity level;
- -
- A 5 times increase of the Vickers micro-hardness was correlated with an increase in roughness, thickness and crystalline phase composition of the coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A







References
- Moosbrugger, C. Engineering Properties of Magnesium Alloys; ASM International: Almere, The Netherlands, 2017; pp. 1–12. [Google Scholar]
- Trang, T.T.T.; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.-C.; Kim, N.J. Designing a magnesium alloy with high strength and high formability. Nat. Commun. 2018, 9, 2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manroo, S.A.; Khan, N.Z.; Ahmad, B. Study on surface modification and fabrication of surface composites of magnesium alloys by friction stir processing: A review. J. Eng. Appl. Sci. 2022, 69, 25. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma electrolytic oxidation of AZ31 magnesium alloy in aluminate-tungstate electrolytes and the coating formation mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Predko, P.; Rajnovic, D.; Grilli, M.; Postolnyi, B.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr: A Recent Progress Review. Metals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Kaseem, M.; Dikici, B. Optimization of Surface Properties of Plasma Electrolytic Oxidation Coating by Organic Additives: A Review. Coatings 2021, 11, 374. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface modification of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses. J. Magnes. Alloy. 2022, 10, 2025–2061. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Xiong, H.; Zhou, J.; Yu, H.; Qin, C.; Wang, Z. Stearic Acid Coated MgO Nanoplate Arrays as Effective Hydrophobic Films for Improving Corrosion Resistance of Mg-Based Metallic Glasses. Nanomaterials 2020, 10, 947. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2020, 117, 100735. [Google Scholar] [CrossRef]
- Štrbák, M.; Kajánek, D.; Knap, V.; Florková, Z.; Pastorková, J.; Hadzima, B.; Goraus, M. Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment. Coatings 2022, 12, 566. [Google Scholar] [CrossRef]
- Esmaeili, M.; Tadayonsaidi, M.; Ghorbanian, B. The effect of PEO parameters on the properties of biodegradable Mg alloys: A review. Surf. Innov. 2021, 9, 184–198. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Plasma Electrolytic Oxidation Coatings on Mg-Alloys for Improved Wear and Corrosion Resistance. Corros. Mater. Perform. Cathodic Prot. 2017, 99, 133–147. [Google Scholar]
- Fattah-Alhosseini, A.; Chaharmahali, R.; Babaei, K. Impressive strides in amelioration of corrosion and wear behaviors of Mg alloys using applied polymer coatings on PEO porous coatings: A review. J. Magnes. Alloy. 2022, 10, 1171–1190. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-Alhosseini, A.; Nouri, M.; Babaei, K. Improving surface characteristics of PEO coatings of Mg and its alloys with zirconia nanoparticles: A review. Appl. Surf. Sci. Adv. 2021, 6, 100131. [Google Scholar] [CrossRef]
- Brady, M.P.; Leonard, D.N.; McNally, E.A.; Kish, J.R.; Meyer, H.M.; Cakmak, E.; Davis, B. Magnesium Alloy Effects on Plasma Electrolytic Oxidation Electro-Ceramic and Electro-Coat Formation and Corrosion Resistance. J. Electrochem. Soc. 2019, 166, C492–C508. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, W.; Zhang, T.; Wang, F. Effect of variations of Al content on microstructure and corrosion resistance of PEO coatings on Mg Al alloys. J. Alloys Compd. 2017, 690, 195–205. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Sun, H.; Li, Y. Effect of heat treatment on corrosion behavior of AZ63 magnesium alloy in 3.5 wt.% sodium chloride solution. Corros. Sci. 2016, 111, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Incesu, A.; Güngör, A. Effect of different heat treatment conditions on microstructural and mechanical behaviour of AZ63 magnesium alloy. Adv. Mater. Process. Technol. 2015, 1, 243–253. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Tang, G.; Chu, P.K. Influence of heat treatment on degradation behavior of bio-degradable die-cast AZ63 magnesium alloy in simulated body fluid. Mater. Sci. Eng. A 2007, 456, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Jafari, H.; Idris, M.; Ourdjini, A.; Payganeh, G. Effect of thermomechanical treatment on microstructure and hardness behavior of AZ63 magnesium alloy. Acta Met. Sin. (Eng. Lett.) 2009, 22, 401–407. [Google Scholar] [CrossRef]
- Khokhlova, J.; Khokhlov, M.; Synyuk, V. Magnesium alloy AZ63A reinforcement by alloying with gallium and using high-disperse ZrO2 particles. J. Magnes. Alloy. 2016, 4, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cao, Z.; Liu, Y.; Su, G.; Cheng, L. Effect of Al content on the microstructures and mechanical properties of Mg–Al alloys. Mater. Sci. Eng. A 2009, 508, 129–133. [Google Scholar] [CrossRef]
- Pei, Y.; Gui, Y.; Huang, T.; Chen, F.; Guo, J.; Zhong, S.; Song, Z. Microstructure and corrosion behaviors of AZ63 magnesium alloy fabricated by accumulative roll bonding process. Mater. Res. Express 2020, 7, 066525. [Google Scholar] [CrossRef]
- Krishna, L.R.; Poshal, G.; Jyothirmayi, A.; Sundararajan, G. Relative hardness and corrosion behavior of micro arc oxidation coatings deposited on binary and ternary magnesium alloys. Mater. Des. 2015, 77, 6–14. [Google Scholar] [CrossRef]
- IPAD Website. Available online: https://ipad.ro/ (accessed on 1 November 2022).
- Malinovschi, V.; Marin, A.H.; Ducu, C.; Moga, S.; Andrei, V.; Coaca, E.; Craciun, V.; Lungu, M.; Lungu, C.P. Improvement of Mechanical and Corrosion Properties of Commercially Pure Titanium Using Alumina PEO Coatings. Coatings 2021, 12, 29. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Curran, J. Thermal and Mechanical Properties of Plasma Electrolytic Oxide Coatings. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2005. [Google Scholar]
- Yao, Z.; Jiang, Y.; Jia, F.; Jiang, Z.; Wang, F. Growth characteristics of plasma electrolytic oxidation ceramic coatings on Ti–6Al–4V alloy. Appl. Surf. Sci. 2008, 254, 4084–4091. [Google Scholar] [CrossRef]
- Aliasghari, S.; Němcová, A.; Skeldon, P.; Thompson, G. Influence of coating morphology on adhesive bonding of titanium pre-treated by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 289, 101–109. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; O Northwood, D.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Babu, N.K.; Krishna, L.R.; Rameshbabu, N. Influence of frequency and duty cycle on microstructure of plasma electrolytic oxidized AA7075 and the correlation to its corrosion behavior. Surf. Coat. Technol. 2015, 280, 136–147. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F.; Rouhaghdam, A.S. Formation of a compact oxide layer on AZ91D magnesium alloy by microarc oxidation via addition of cerium chloride into the MAO electrolyte. J. Coat. Technol. Res. 2011, 8, 765–771. [Google Scholar] [CrossRef]
- ISO 25178-2; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2012.
- Mojsilović, K.; Lačnjevac, U.; Stojanović, S.; Damjanović-Vasilić, L.; Stojadinović, S.; Vasilić, R. Formation and Properties of Oxide Coatings with Immobilized Zeolites Obtained by Plasma Electrolytic Oxidation of Aluminum. Metals 2021, 11, 1241. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Sieber, M.; Simchen, F.; Scharf, I.; Lampke, T. Formation of a Spinel Coating on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation. J. Mater. Eng. Perform. 2016, 25, 1157–1162. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Luan, B.L. Corrosion and wear properties of PEO coatings formed on AM60B alloy in NaAlO2 electrolytes. Appl. Surf. Sci. 2011, 257, 9135–9141. [Google Scholar] [CrossRef]
- Ganesh, I. A Review on Magnesium Aluminate (MgAl2O4) Spinel: Synthesis, Processing and Applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Wei, Y.; Gu, S.; Fang, H.; Luo, W.; Zhang, X.; Wang, L.; Jiang, W. Properties of MgO transparent ceramics prepared at low temperature using high sintering activity MgO powders. J. Am. Ceram. Soc. 2020, 103, 5382–5391. [Google Scholar] [CrossRef]
- Xue, W.; Deng, Z.; Chen, R.; Zhang, T. Growth regularity of ceramic coatings formed by microarc oxidation on Al–Cu–Mg alloy. Thin Solid Film 2000, 372, 114–117. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Li, H.; Lu, Y.; Yu, H.; Chen, C. Enhanced corrosion resistance of magnesium alloy by plasma electrolytic oxidation plus hydrothermal treatment. Surf. Coat. Technol. 2021, 424, 127662. [Google Scholar] [CrossRef]
- Toulabifard, A.; Rahmati, M.; Raeissi, K.; Hakimizad, A.; Santamaria, M. The Effect of Electrolytic Solution Composition on the Structure, Corrosion, and Wear Resistance of PEO Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 937. [Google Scholar] [CrossRef]











| Sample Code | Porosity ± SD (%) |
|---|---|
| AZ63_5m | 19.17 ± 1.96 |
| AZ63_10m | 14.59 ± 1.40 |
| AZ63_20m | 11.30 ± 1.36 |
| Sample Code | Sq ± SD (µm) | Sa ± SD (μm) |
|---|---|---|
| AZ63_5m | 5.11 ± 2.08 | 4.28 ± 2.04 |
| AZ63_10m | 5.24 ± 0.36 | 4.17 ± 0.29 |
| AZ63_20m | 6.67 ± 1.29 | 5.23 ± 1.03 |
| Element ± SD (wt%) | ||||
|---|---|---|---|---|
| Mg | Al | Zn | Mn | Si |
| 91.55 ± 0.05 | 5.36 ± 0.04 | 2.83 ± 0.03 | 0.17 ± 0.01 | 0.09 ± 0.01 |
| Sample | Point Scan P1 | Point Scan P2 | Point Scan P3 | Point Scan P4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg (wt%) | Al (wt%) | O (wt%) | Mg (wt%) | Al (wt%) | O (wt%) | Mg (wt%) | Al (wt%) | O (wt%) | Mg (wt%) | Al (wt%) | O (wt%) | |
| AZ63_5m | 23.70 ± 0.11 | 30.85 ± 0.14 | 45.44 ± 0.17 | 22.19 ± 0.09 | 32.40 ± 0.20 | 47.36 ± 0.11 | 24.08 ± 0.06 | 30.70 ± 0.08 | 45.22 ± 0.09 | 23.68 ± 0.05 | 30. 59 ± 0.09 | 45.73 ± 0.08 |
| AZ63_10m | 26.43 ± 0.13 | 29.35 ± 0.14 | 44.22 0.17± | 27.11 ± 0.13 | 28.53 ± 0.14 | 44.35 ± 0.17 | 32.40 ± 0.05 | 25.16 ± 0.05 | 42.44 ± 0.06 | 27.46 ± 0.05 | 31.53 ± 0.06 | 41.01 ± 0.07 |
| AZ63_20m | 20.38 ± 0.10 | 33.18 ± 0.12 | 46.44 ± 0.15 | 24.23 ± 0.12 | 30.71 ± 0.14 | 45. 06 ± 0.17 | 25.23 ± 0.06 | 29.88 ± 0.08 | 44.89 ± 0.09 | 23.00 ± 0.04 | 33.47 ± 0.05 | 43.53 ± 0.06 |
| Sample | Coating Thickness (µm) | Barrier Layer Thickness (µm) |
|---|---|---|
| AZ63_5m | 15.14 ± 4.21 | 0.36 ± 0.06 |
| AZ63_10m | 23.78 ± 11.64 | 0.66 ± 0.08 |
| AZ63_20m | 36.60 ± 9.22 | 0.67 ± 0.05 |
| Sample | Phase Composition | Quantitative Phase Composition (wt%)—Rietveld Analysis | Crystallite Mean Size (nm) |
|---|---|---|---|
| AZ63_5m | MgAl2O4 | 82.2 ± 0.4 | 35.9 ± 1.6 |
| MgO | 7.5 ± 0.4 | NA | |
| gamma-Al0.667O | 10.3 ± 1.2 | NA | |
| AZ63_10m | MgAl2O4 | 89.2 ± 0.3 | 33.3 ± 0.3 |
| MgO | 7.1 ± 0.4 | NA | |
| gamma-Al0.667O | 3.7 ± 0.5 | NA | |
| AZ63_20m | MgAl2O4 | 91.2 ± 0.2 | 35.0 ± 0.3 |
| MgO | 4.9 ± 0.2 | NA | |
| gamma-Al0.667O | 3.8 ± 0.2 | NA |
| Sample | HV/0.3 (GPA) |
|---|---|
| AZ63 | 0.88 ± 0.09 |
| AZ63_5m | 2.04 ± 0.09 |
| AZ63_10m | 3.42 ± 0.17 |
| AZ63_20m | 4.44 ± 0.12 |
| Sample | βa (mV) | βc (mV) | Ecorr (V vs. SCE) | icorr (A/cm2) | Vcorr (mmpy) |
|---|---|---|---|---|---|
| AZ63 | 20.6 | 222.7 | −1.49 | 51.0 × 10−5 | 11.1 |
| AZ63_5m | 77.85 | 404.8 | −1.48 | 15.6 × 10−5 | 4.0 |
| AZ63_10m | 30.01 | 647.5 | −1.35 | 2.91 × 10−5 | 2.4 |
| AZ63_20m | 37.56 | 419.04 | −1.39 | 0.83 × 10−5 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, S.G.; Negrea, D.A.; Ducu, C.M.; Malinovschi, V.; Schiopu, A.G.; Coaca, E.; Patrascu, I. The Influence of Processing Time on Morphology, Structure and Functional Properties of PEO Coatings on AZ63 Magnesium Alloy. Appl. Sci. 2022, 12, 12848. https://doi.org/10.3390/app122412848
Moga SG, Negrea DA, Ducu CM, Malinovschi V, Schiopu AG, Coaca E, Patrascu I. The Influence of Processing Time on Morphology, Structure and Functional Properties of PEO Coatings on AZ63 Magnesium Alloy. Applied Sciences. 2022; 12(24):12848. https://doi.org/10.3390/app122412848
Chicago/Turabian StyleMoga, Sorin Georgian, Denis Aurelian Negrea, Catalin Marian Ducu, Viorel Malinovschi, Adriana Gabriela Schiopu, Elisabeta Coaca, and Ion Patrascu. 2022. "The Influence of Processing Time on Morphology, Structure and Functional Properties of PEO Coatings on AZ63 Magnesium Alloy" Applied Sciences 12, no. 24: 12848. https://doi.org/10.3390/app122412848