Role of Förster Resonance Energy Transfer in Graphene-Based Nanomaterials for Sensing
Abstract
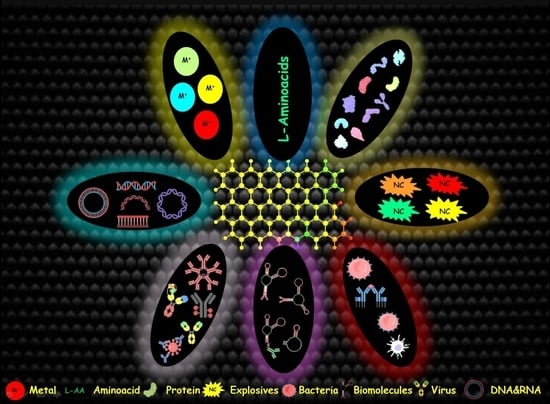
:1. Introduction
2. Origin of Fluorescence in Graphene-Based Nanomaterials and the FRET Process
Förster Resonance Energy Transfer (FRET)
3. Application of Graphene-Based Nanomaterials in Sensing
3.1. Metal Ion Sensor
3.2. Explosive Sensor
3.3. H2O2 Sensor
3.4. Theophylline Sensor
3.5. Glutathione Sensor
3.6. Proteins (Cancer Biomarkers) Sensor
3.7. Biomolecule, Bacteria, and Virus Sensor
3.8. DNA and RNA Sensor
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kadian, S.; Sethi, S.K.; Manik, G. Recent advancements in synthesis and property control of graphene quantum dots for biomedical and optoelectronic applications. Mater. Chem. Front. 2021, 5, 627–658. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. Advances in Gold Nanoparticles-Based Colorimetric Aptasensors for the Detection of Antibiotics: An Overview of the Past Decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Yim, Y.; Shin, H.; Ahn, S.M.; Min, D.H. Graphene oxide-based fluorescent biosensors and their biomedical applications in diagnosis and drug discovery. Chem. Commun. 2021, 57, 9820–9833. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.U.A.; Fang, X.; Luo, Z.; Ullah, S.; Fatima, S.; Batool, S.; Qiu, B.; Shahzad, F. Graphene Based Nanohybrid Aptasensors in Environmental Monitoring: Concepts, Design and Future Outlook. Crit. Rev. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]
- Milosavljevic, V.; Mitrevska, K.; Adam, V. Benefits of oxidation and size reduction of graphene/graphene oxide nanoparticles in biosensing application: Classification of graphene/graphene oxide nanoparticles. Sensors Actuators B Chem. 2022, 353, 131122. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Hunt, A.; Dikin, D.A.; Kurmaev, E.Z.; Boyko, T.D.; Bazylewski, P.; Chang, G.S.; Moewes, A. Epoxide Speciation and Functional Group Distribution in Graphene Oxide Paper-Like Materials. Adv. Funct. Mater. 2012, 22, 3950–3957. [Google Scholar] [CrossRef]
- Benjamin, C.B. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 2002, 80, 1339. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Der Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Imani, M.; Mohajeri, N.; Rastegar, M.; Zarghami, N. Recent advances in FRET-Based biosensors for biomedical applications. Anal. Biochem. 2021, 630, 114323. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ren, B.; Zhang, D.; Liu, Y.; Zhang, M.; Zhao, C.; Zheng, J. Design principles and fundamental understanding of biosensors for amyloid-β detection. J. Mater. Chem. B 2020, 8, 6179–6196. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Xia, S.; Wang, X.; Yu, Y.; Zhou, H.; Liu, H. A FRET-based near-infrared ratiometric fluorescent probe for detection of mitochondria biothiol. Talanta 2020, 219, 121296. [Google Scholar] [CrossRef]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Ghosh, A.; Chizhik, A.I.; Karedla, N.; Enderlein, J. Graphene- and metal-induced energy transfer for single-molecule imaging and live-cell nanoscopy with (sub)-nanometer axial resolution. Nat. Protoc. 2021, 16, 3695–3715. [Google Scholar] [CrossRef]
- He, L.; Lu, D.Q.; Liang, H.; Xie, S.; Luo, C.; Hu, M.; Xu, L.; Zhang, X.; Tan, W. Fluorescence Resonance Energy Transfer-Based DNA Tetrahedron Nanotweezer for Highly Reliable Detection of Tumor-Related mRNA in Living Cells. ACS Nano 2017, 11, 4060–4066. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.J.; Min, D.H. Emerging Approaches for Graphene Oxide Biosensor. Anal. Chem. 2016, 89, 232–248. [Google Scholar] [CrossRef]
- Pehlivan, Z.S.; Torabfam, M.; Kurt, H.; Ow-Yang, C.; Hildebrandt, N.; Yüce, M. Aptamer and nanomaterial based FRET biosensors: A review on recent advances (2014–2019). Microchim. Acta 2019, 186, 563. [Google Scholar] [CrossRef]
- Su, S.; Chen, S.; Fan, C. Recent advances in two-dimensional nanomaterials-based electrochemical sensors for environmental analysis. Green Energy Environ. 2018, 3, 97–106. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Suguna, S.; David, C.I.; Prabhu, J.; Nandhakumar, R. Functionalized graphene oxide materials for the fluorometric sensing of various analytes: A mini review. Mater. Adv. 2021, 2, 6197–6212. [Google Scholar] [CrossRef]
- Ueno, Y. Graphene-Based FRET Aptasensors. Anal. Sci. 2021, 37, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Huang, L.; Qu, H.; Han, Z.; Wang, Y.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Review of performance improvement strategies for doped graphene quantum dots for fluorescence-based sensing. Synth. Met. 2021, 276, 116758. [Google Scholar] [CrossRef]
- Ansari, A.A.; Thakur, V.K.; Chen, G. Functionalized upconversion nanoparticles: New strategy towards FRET-based luminescence bio-sensing. Coord. Chem. Rev. 2021, 436, 213821. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Cristina, G.N.; Sundaram, R.S.; Burghard, M.; Kern, K. Electrical Conduction Mechanism in Chemically Derived Graphene Monolayers. Nano Lett. 2009, 9, 1787–1792. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.Y.; Miller, S.; Chen, C.W.; Su, W.F.; Chhowalla, M. Transparent and conducting electrodes for organic electronics from reduced graphene oxide. Appl. Phys. Lett. 2008, 92, 233305. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Zhou, X.; Guo, S.; Wu, N. Fingerprinting photoluminescence of functional groups in graphene oxide. J. Mater. Chem. 2012, 22, 23374–23379. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
- Swathi, R.S.; Sebastian, K.L. Resonance energy transfer from a dye molecule to graphene. J. Chem. Phys. 2008, 129, 054703. [Google Scholar] [CrossRef]
- Swathi, R.S.; Sebastian, K.L. Long range resonance energy transfer from a dye molecule to graphene has (distance)−4 dependence. J. Chem. Phys. 2009, 130, 086101. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Pang, W.; Yan, G. An aptamer-based fluorescence probe for facile detection of lipopolysaccharide in drinks. RSC Adv. 2017, 7, 54920–54926. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. –Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef]
- Gilboa, T.; Garden, P.M.; Cohen, L. Single-molecule analysis of nucleic acid biomarkers—A review. Anal. Chim. Acta 2020, 1115, 61–85. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef]
- Johnson, A.P.; Sabu, C.; Swamy, N.K.; Anto, A.; Gangadharappa, H.V.; Pramod, K. Graphene nanoribbon: An emerging and efficient flat molecular platform for advanced biosensing. Biosens. Bioelectron. 2021, 184, 113245. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Rauf, S.; Beduk, T.; Durmus, C.; Aljedaibi, A.; Timur, S.; Alshareef, H.N.; Amine, A.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors and biosensors using laser-derived graphene: A comprehensive review. Biosens. Bioelectron. 2020, 168, 112565. [Google Scholar] [CrossRef] [PubMed]
- Siefe, C.; Mehlenbacher, R.D.; Peng, C.S.; Zhang, Y.; Fischer, S.; Lay, A.; McLellan, C.A.; Alivisatos, A.P.; Chu, S.; Dionne, J.A. Sub-20 nm Core-Shell-Shell Nanoparticles for Bright Upconversion and Enhanced Förster Resonant Energy Transfer. J. Am. Chem. Soc. 2019, 141, 16997–17005. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence Resonant Energy Transfer Biosensor Based on Upconversion-Luminescent Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiao, B. DNA covalently linked to graphene oxide for biotin–streptavidin interaction assay. Talanta 2017, 163, 140–145. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, Z.H.; Xue, S.F.; Han, X.Y.; Lin, Z.Y.; Zhang, S.; Shi, G.; Zhang, M. Lanthanide-doped nanoparticles encountering porphyrin hydrate: Boosting a dual-mode optical nanokit for Cu2+ sensing. Sensors Actuators B Chem. 2018, 268, 108–114. [Google Scholar] [CrossRef]
- Tu, D.; Liu, L.; Ju, Q.; Liu, Y.; Zhu, H.; Li, R.; Chen, X. Time-Resolved FRET Biosensor Based on Amine-Functionalized Lanthanide-Doped NaYF4 Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 6306–6310. [Google Scholar] [CrossRef]
- Jung, J.H.; Cheon, D.S.; Liu, F.; Lee, K.B.; Seo, T.S. A Graphene Oxide Based Immuno-biosensor for Pathogen Detection. Angew. Chem. 2010, 122, 5844–5847. [Google Scholar] [CrossRef]
- Hai, X.; Li, Y.; Yu, K.; Yue, S.; Li, Y.; Song, W.; Bi, S.; Zhang, X. Synergistic in-situ growth of silver nanoparticles with nanozyme activity for dual-mode biosensing and cancer theranostics. Chin. Chem. Lett. 2021, 32, 1215–1219. [Google Scholar] [CrossRef]
- Soundharraj, P.; Dhinasekaran, D.; Aruna, P.; Ganesan, S. Facile synthesis of biomass silica-silver colloidal nanoparticles and its application as highly sensitive fluorescent biosensor. Surf. Interfaces 2021, 23, 101010. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Lou, Z.; Zeng, L.; Zhu, M. Molecularly imprinted photoelectrochemical sensor for detecting tetrabromobisphenol A in indoor dust and water. Microchim. Acta 2021, 188, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, M. Detection of pollutants in water bodies: Electrochemical detection or photo-electrochemical detection? Chem. Commun. 2020, 56, 14541–14552. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshbin, Z.; Housaindokht, M.R.; Izadyar, M.; Verdian, A.; Bozorgmehr, M.R. A simple paper-based aptasensor for ultrasensitive detection of lead (II) ion. Anal. Chim. Acta 2019, 1071, 70–77. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.R.; Verdian, A. A low-cost paper-based aptasensor for simultaneous trace-level monitoring of mercury (II) and silver (I) ions. Anal. Biochem. 2020, 597, 113689. [Google Scholar] [CrossRef]
- Mandal, D.; Khatun, S.; Gupta, A.N.; Chandra, A. DNA supported graphene quantum dots for Ag ion sensing. Nanotechnology 2019, 30, 255501. [Google Scholar] [CrossRef] [Green Version]
- Mo, F.; Ma, Z.; Wu, T.; Liu, M.; Zhang, Y.; Li, H.; Yao, S. Holey reduced graphene oxide inducing sensitivity enhanced detection nanoplatform for cadmium ions based on glutathione-gold nanocluster. Sensors Actuators B Chem. 2019, 281, 486–492. [Google Scholar] [CrossRef]
- Asha, J.B.; Suresh, P. Covalently Modified Graphene Oxide as Highly Fluorescent and Sustainable Carbonaceous Chemosensor for Selective Detection of Zirconium ion in Complete Aqueous Medium. ACS Sustain. Chem. Eng. 2020, 8, 14301–14311. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Bezerra, M.L.S.; Neves, E.B.; Moreira, F.R. Impact of ammunition and military explosives on human health and the environment. Rev. Environ. Health 2011, 26, 101–110. [Google Scholar] [CrossRef]
- Tian, R.; Ji, P.; Wang, L.; Zhang, H.; Sun, J. TNT sensor based on accumulation layer and effective distance of FRET mechanism with ultra-high sensitivity. Microchem. J. 2021, 160, 105706. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Chen, S.; Lin, Y.; Lai, H.; Chen, B.; Chen, T. Biomedical Application of Reactive Oxygen Species–Responsive Nanocarriers in Cancer, Inflammation, and Neurodegenerative Diseases. Front. Chem. 2020, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.L.; Song, X.; Niu, C.B.; Lv, Q.Y.; Li, C.L.; Cui, H.F.; Zhang, S. Red fluorescent nanoprobe based on Ag@Au nanoparticles and graphene quantum dots for H2O2 determination and living cell imaging. Microchim. Acta 2021, 188, 291. [Google Scholar] [CrossRef] [PubMed]
- Hallworth, M. Therapeutic drug monitoring. Clin. Biochem. Metab. Clin. Asp. Third Ed. 2014, 767–786. [Google Scholar] [CrossRef]
- Wu, J.F.; Gao, X.; Ge, L.; Zhao, G.C.; Wang, G.F. A fluorescence sensing platform of theophylline based on the interaction of RNA aptamer with graphene oxide. RSC Adv. 2019, 9, 19813–19818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Wang, X.; Dong, C.; Shuang, S. Graphene quantum dots wrapped square-plate-like MnO2 nanocomposite as a fluorescent turn-on sensor for glutathione. Talanta 2020, 219, 121180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yan, X.; Qiu, J.; Sun, J.; Zhao, X.E. Turn-on fluorescent assay for antioxidants based on their inhibiting polymerization of dopamine on graphene quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117516. [Google Scholar] [CrossRef]
- Bharathi, G.; Lin, F.; Liu, L.; Ohulchanskyy, T.Y.; Hu, R.; Qu, J. An all-graphene quantum dot Förster resonance energy transfer (FRET) probe for ratiometric detection of HE4 ovarian cancer biomarker. Colloids Surfaces B Biointerfaces 2021, 198, 111458. [Google Scholar] [CrossRef]
- Youn, H.; Lee, K.; Her, J.; Jeon, J.; Mok, J.; So, J.-i.; Shin, S.; Ban, C. Aptasensor for multiplex detection of antibiotics based on FRET strategy combined with aptamer/graphene oxide complex. Sci. Rep. 2019, 9, 7659. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, Z.; Mohammadnejad, J.; Hosseini, M.; bakhshi, B.; Rezayan, A.H. Whole cell FRET immunosensor based on graphene oxide and graphene dot for Campylobacter jejuni detection. Food Chem. 2020, 309, 125690. [Google Scholar] [CrossRef]
- Elmizadeh, H.; Faridbod, F.; Soleimani, M.; Ganjali, M.R.; Bardajee, G.R. Fluorescent apta-nanobiosensors for fast and sensitive detection of digoxin in biological fluids using rGQDs: Comparison of two approaches for immobilization of aptamer. Sensors Actuators B Chem. 2020, 302, 127133. [Google Scholar] [CrossRef]
- Kamal, Z.; Zarei Ghobadi, M.; Mohseni, S.M.; Ghourchian, H. High-performance porphyrin-like graphene quantum dots for immuno-sensing of Salmonella typhi. Biosens. Bioelectron. 2021, 188, 113334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Du, P.; Wang, X.; Huang, M.; Sun, L.D.; Wang, T.; Wang, Z. Upconversion Fluorescence Resonance Energy Transfer Aptasensors for H5N1 Influenza Virus Detection. ACS Omega 2021, 6, 15236–15245. [Google Scholar] [CrossRef] [PubMed]
- Bahari, D.; Babamiri, B.; Salimi, A.; Rashidi, A. Graphdiyne/graphene quantum dots for development of FRET ratiometric fluorescent assay toward sensitive detection of miRNA in human serum and bioimaging of living cancer cells. J. Lumin. 2021, 239, 118371. [Google Scholar] [CrossRef]
- Balaji, A.; Yang, S.; Wang, J.; Zhang, J. Graphene Oxide-Based Nanostructured DNA Sensor. Biosensors 2019, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.T.; Nguyen, V.-T.; Kim, M.-S. Graphene Oxide-Based Simple and Rapid Detection of Antibiotic Resistance Gene via Quantum Dot-Labeled Zinc Finger Proteins. Anal. Chem. 2021, 93, 8459–8466. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Z.; Song, Z.; Xiao, L.; He, Y. A strategy for preparing non-fluorescent graphene oxide quantum dots as fluorescence quenchers in quantitative real-time PCR. RSC Adv. 2020, 10, 14944–14952. [Google Scholar] [CrossRef] [Green Version]























| S.No | Sensors | Analytes | Mechanism | Binding Constant (M−1) | LOD (M) | Ref. |
|---|---|---|---|---|---|---|
| 1 | GO aptasensor | Pb2+ | FRET | 0.07–20 nM. | 0.5 pM | [54] |
| 2 | GO aptasensor | Ag+ and Hg2+ | FRET | 0.05–50 nM | 1.33 pM | [55] |
| 3 | GQD (NGQD) | Ag+ | FRET | - | 0.05–0.02 nM | [56] |
| 4 | GSH-AuNCs | Cd2+ | FRET | 0.1–100 µM | 42.0 nM | [57] |
| 5 | GO | Zr4+ | FRET | 1.7 × 104 M−1 | 27 ng/mL | [58] |
| 6 | GQDs | TNT | FRET | 1–20 nM | 0.6 nM | [60] |
| 7 | Ag@AuNPs | H2O2 | FRET | - | 0.4 µM | [62] |
| 8 | GO-QDs | Theophylline | FRET | - | 4 nM | [64] |
| 9 | GQDs-MnO2 | Glutathione | FRET | - | 48 nM | [66] |
| 10 | GQDs | Glutathione | FRET | 0.01–0.1 µM 0.1–35 µM | 2.4 nM | [67] |
| FRET | 0.01–0.1 µM 0.1–25 µM | 1.5 nM | ||||
| FRET | 0.01–0.1 µM 0.1–30 µM | 4.2 nM | ||||
| FRET | 0.01–0.1 µM 0.1–30 µM | 4.4 nM | ||||
| 11 | GQDs | HE4 (Protein) | FRET | - | 4.8 pM–300 nM | [68] |
| 12 | GO/aptamer | Antibiotics | FRET | - | (i) 1.997 ng/mL (ii) 2.664 ng/mL (iii) 2.337 ng/mL | [69] |
| 13 | GQDs | Campylobacter jejuni | FRET | 10–106 CFU | 10 CFU/mL | [70] |
| 14 | rGQDs | Digoxin | FRET | (i) 9.95 ± 0.32 × 10−11 mol L−1 (ii) 0.54 ± 0.02 × 10−9 mol L−1 | (i) 29.87 ± 1.01 × 10−12 mol L−1 (ii) 7.95 ± 0.22 × 10−12 mol L−1 | [71] |
| 15 | GQDs | Vi antigen | FRET | - | 1 pg/mL | [72] |
| 16 | GO/HA aptamer | H5N1 IAV hemagglutinin (HA) | FRET | 0.1–15 ng mL−1 | 60.9 pg mL−1 | [73] |
| 17 | GDQDs | miRNA-21 | FRET | 5 pM–200 nM | 0.5 pM | [74] |
| 18 | GO | DNA-t and DNA-c | FRET | - | 0.12 µM | [75] |
| 19 | QD/GO | DNA-t | FRET | - | 1 nM | [76] |
| 20 | GOQDs | DNA | FRET | - | 104 –1010 µL | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabakaran, G.; Velmurugan, K.; David, C.I.; Nandhakumar, R. Role of Förster Resonance Energy Transfer in Graphene-Based Nanomaterials for Sensing. Appl. Sci. 2022, 12, 6844. https://doi.org/10.3390/app12146844
Prabakaran G, Velmurugan K, David CI, Nandhakumar R. Role of Förster Resonance Energy Transfer in Graphene-Based Nanomaterials for Sensing. Applied Sciences. 2022; 12(14):6844. https://doi.org/10.3390/app12146844
Chicago/Turabian StylePrabakaran, G., K. Velmurugan, C. Immanuel David, and R. Nandhakumar. 2022. "Role of Förster Resonance Energy Transfer in Graphene-Based Nanomaterials for Sensing" Applied Sciences 12, no. 14: 6844. https://doi.org/10.3390/app12146844