Nanostructured Na2CaP2O7: A New and Efficient Catalyst for One-Pot Synthesis of 2-Amino-3-Cyanopyridine Derivatives and Evaluation of Their Antibacterial Activity
Abstract
:1. Introduction
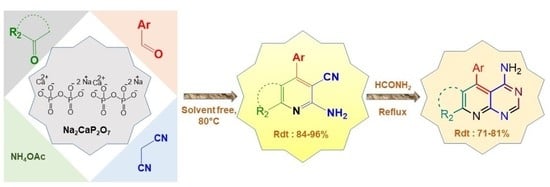
2. Results and Discussion
2.1. Synthesis and Characterization of Na2CaP2O7 Nanoparticles
2.2. Characterization of Diphosphate Na2CaP2O7
2.3. Optimization of Reaction Conditions
2.4. Influence of the Amount of the Catalyst
2.5. Influence of Reaction Time
2.6. Influence of the Solvent
2.7. Recyclability of Na2CaP2O7 Catalyst
2.8. Antimicrobial Activity
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, M.D. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem. Eur. J. 2010, 16, 12052–12062. [Google Scholar] [CrossRef] [PubMed]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar]
- Hamada, Y. Role of Pyridines in Medicinal Chemistry and Design of BACE1 Inhibitors Possessing a Pyridine Scaffold; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Radwan, M.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Hemdan, B.A.; El-Kady, D.S. Synthesis, Molecular Docking and Antimicrobial Activity of New Fused Pyrimidine and Pyridine Derivatives. Bioorgan. Chem. 2020, 96, 103516. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Almahli, H.; Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A. Pyridine-Ureas as Potential Anticancer Agents: Synthesis and in Vitro Biological Evaluation. Molecules 2018, 23, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Faheem; Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine-and Thiazole-Based Hydrazides with Promising Anti-Inflammatory and Antimicrobial Activities along with Their in Silico Studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Antiviral Activities of Pyridine Fused and Pyridine Containing Heterocycles, A Review (from 2000 to 2020). Mini Rev. Med. Chem. 2021, 21, 2584–2611. [Google Scholar] [CrossRef]
- Sadawarte, G.; Jagatap, S.; Patil, M.; Jagrut, V.; Rajput, J.D. Synthesis of Substituted Pyridine Based Sulphonamides as an Antidiabetic Agent. Eur. J. Chem. 2021, 12, 279–283. [Google Scholar] [CrossRef]
- Khafagy, M.M.; Abd El-Wahab, A.H.F.; Eid, F.A.; El-Agrody, A.M. Synthesis of Halogen Derivatives of Benzo[h]Chromene and Benzo[a]Anthracene with Promising Antimicrobial Activities. Il Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- Hu, H.; Peng, Y.; Huang, H.; Yang, T.; Chen, F.; Yan, P. Deacylation during the Synthesis of New 4-Amino-1H-Pyrazolo [3,4-B] Pyridines Catalysed by Sncl4. J. Chem. Res. 2018, 42, 412–415. [Google Scholar] [CrossRef]
- Potapov, A.Y.; Vandyshev, D.Y.; Kosheleva, Y.A.; Polikarchuk, V.A.; Potapov, M.A.; Shikhaliev, K.S. Cyclization of 5-Amino-1-Aryl-1H-Pyrazole-4-Carbonitriles with β-Dicarbonyl Compounds. Chem. Heterocycl. Compd. 2017, 53, 207–212. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Abd El Ghani, G.E.; Khalil, A.E.-G.M. Chemistry of 2-Amino-3-Cyanopyridines. Synth. Commun. 2014, 44, 297–330. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-Free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, L.; Yao, Y.; Zhang, L.; Wang, W. One-Pot Synthesis of 2-Amino-3-Cyanopyridine Derivatives Catalyzed by Ytterbium Perfluorooctanoate [Yb(PFO)3]. Tetrahedron Lett. 2011, 52, 509–511. [Google Scholar] [CrossRef]
- Kurumurthy, C.; Naresh Kumar, R.; Yakaiah, T.; Shanthan Rao, P.; Narsaiah, B. Novel Bu4N+Br− Catalyzed One-Pot Multi-Component Synthesis of 2-Amino Nicotinonitriles in Aqueous Medium. Res. Chem. Intermed. 2015, 41, 3193–3199. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Abbaspour-Gilandeh, E.; Aghaei-Hashjin, M. Four-Component Synthesis of 2-Amino-3-Cyanopyridine Derivatives Catalyzed by Cu@imineZCMNPs as a Novel, Efficient and Simple Nanocatalyst Under Solvent-Free Conditions. Catal. Lett. 2018, 148, 1254–1262. [Google Scholar] [CrossRef]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, P.N.; Malik, S. Aqueous Media Preparation of 2-Amino-4,6-Diphenylnicotinonitriles Using Cellulose Sulfuric Acid as an Efficient Catalyst. Res. Chem. Intermed. 2014, 40, 871–885. [Google Scholar] [CrossRef]
- Sheibani, H.; Saidi, K.; Abbasnejad, M.; Derakhshani, A.; Mohammadzadeh, I. A Convenient One-Pot Synthesis and Anxietic Activity of 3-Cyano-2(1H)-Iminopyridines and Halogen Derivatives of Benzo[h]Chromenes. Arab. J. Chem. 2016, 9, S901–S906. [Google Scholar] [CrossRef] [Green Version]
- Zolfigol, M.A.; Kiafar, M.; Yarie, M.; Taherpour, A.; Fellowes, T.; Nicole Hancok, A.; Yari, A. A Convenient Method for Preparation of 2-Amino-4,6-Diphenylnicotinonitrile Using HBF4 as an Efficient Catalyst via an Anomeric Based Oxidation: A Joint Experimental and Theoretical Study. J. Mol. Struct. 2017, 1137, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Zolfigol, M.A.; Kiafar, M.; Yarie, M.; Taherpour, A.; Saeidi-Rad, M. Experimental and Theoretical Studies of the Nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 Catalyst for 2-Amino-3-Cyanopyridine Preparation via an Anomeric Based Oxidation. RSC Adv. 2016, 6, 50100–50111. [Google Scholar] [CrossRef]
- Zadpour, M.; Behbahani, F.K. Iron(III) Phosphate as a Green and Reusable Catalyst for the Synthesis of 4,6-Disubstituted 2-Aminopyridine-3-Carbonitriles. Mon. Chem. Chem. Mon. 2015, 146, 1865–1869. [Google Scholar] [CrossRef]
- Puligilla Balaswamy, S.A.; Satyanarayana, B. Polyethylene glycol-400 used as phase trasfer catalyst for one-pot synthesis of 2-amino-3cyanopyridine derivates under aqueous conditions. J. Chem. 2017, 10, 1334–1339. [Google Scholar]
- Dänoun, K.; Jioui, I.; Bouhrara, M.; Zahouily, M.; Solhy, A.; Jouiad, M.; Len, C.; Fihri, A. Nano-Structured Pyrophosphate Na2CaP2O7 as Catalyst for Selective Synthesis of 1,2-Disubstituted Benzimidazoles in Pure Water. Curr. Org. Chem. 2015, 19, 2132–2140. [Google Scholar] [CrossRef]
- Maleki, B.; Raei, M.; Alinezhad, H.; Tayebee, R.; Sedrpoushan, A. Chemoselective Synthesis of Tetraketones in Water Catalyzed by Nanostructured Diphosphate Na2CaP2O7. Org. Prep. Proced. Int. 2018, 50, 288–300. [Google Scholar] [CrossRef]
- Maleki, B.; Veisi, H. Facile and Efficient Synthesis of Bicyclic Ortho-Aminocarbonitrile Derivatives Using Nanostructured Diphosphate Na2CaP2O7. Org. Prep. Proced. Int. 2020, 52, 232–237. [Google Scholar] [CrossRef]
- Achagar, R.; Elmakssoudi, A.; Dakir, M.; Elamrani, A.; Zouheir, Y.; Zahouily, M.; Jamaleddine, J. A Green and Efficient Protocol for the Synthesis of Phenylhydrazone Derivatives Catalyzed by Nanostructured Diphosphate Na2CaP2O7 and Screening of Their Antibacterial Activity. ChemistrySelect 2021, 6, 1366–1371. [Google Scholar] [CrossRef]
- Elmakssoudi, A.; Abdelouahdi, K.; Zahouily, M.; Clark, J.; Solhy, A. Efficient Conversion of Aldehydes and Ketones into Oximes Using a Nanostructured Pyrophosphate Catalyst in a Solvent-Free Process. Catal. Commun. 2012, 29, 53–57. [Google Scholar] [CrossRef]
- Zahouily, M.; Elmakssoudi, A.; Mezdar, A.; Rayadh, A.; Sebti, S.; Lazrek, H.B. Three Components Coupling Catalysed by Na2CaP2O7: Synthesis of α-Amino Phosphonates Under Solvent-Free Conditions at Room Temperature. Lett. Org. Chem. 2005, 2, 428–432. [Google Scholar] [CrossRef]
- Solhy, A.; Elmakssoudi, A.; Tahir, R.; Karkouri, M.; Larzek, M.; Bousmina, M.; Zahouily, M. Clean Chemical Synthesis of 2-Amino-Chromenes in Water Catalyzed by Nanostructured Diphosphate Na2CaP2O7. Green Chem. 2010, 12, 2261–2267. [Google Scholar] [CrossRef]
- Addoum, B.; Derdak, R.; Sakoui, S.; Elmakssoudi, A.; Soukri, A. The One-Pot Synthesis of Some Bioactive Pyranopyrazoles and Evaluation of Their Protective Behavior against Extracellular H2O2 and SNP in T. Thermophila. Jordan J. Biol. Sci. 2021, 14, 31–39. [Google Scholar]
- Bennazha, J.; Boukhari, A.; Holt, E.M. Synthesis and Crystal Structure of Na2CaP2O7. Solid State Sci. 1999, 1, 373–380. [Google Scholar] [CrossRef]
- Song, S.-H.; Son, J.-H.; Budiman, A.W.; Choi, M.-J.; Chang, T.-S.; Shin, C.-H. The Influence of Calcination Temperature on Catalytic Activities in a Co Based Catalyst for CO2 Dry Reforming. Korean J. Chem. Eng. 2014, 31, 224–229. [Google Scholar] [CrossRef]
- Tsubota, S.; Nakamura, T.; Tanaka, K.; Haruta, M. Effect of Calcination Temperature on the Catalytic Activity of Au Colloids Mechanically Mixed with TiO2 Powder for CO Oxidation. Catal. Lett. 1998, 56, 131–135. [Google Scholar] [CrossRef]
- Nargund, L.V.G.; Reddy, Y.S.R.; Jose, R. Synthesis and Antibacterial Activity of Pyrido [1,2-a] Pyrimidin-4 (1H)-Ones. Indian Drugs 1991, 29, 45–46. [Google Scholar]
- Mamaghani, M.; Tabatabaeian, K.; Araghi, R.; Fallah, A.; Hossein Nia, R. An Efficient, Clean, and Catalyst-Free Synthesis of Fused Pyrimidines Using Sonochemistry. Org. Chem. Int. 2014, 2014, 406869. [Google Scholar] [CrossRef] [Green Version]
- Salama, M.A.M.; El-Shahat, M.; Elhefny, E.A.; El-Sayed, A.A. A Novel Fused Pyridopyrimidine Derivatives: Synthesis and Characterization. Int. J. Pharm. 2015, 5, 53–58. [Google Scholar]
- Verma, A.K.; Singh, A.K.; Islam, M.M. Synthesis, Characterization and Evaluation of Pyridopyrimidine Carboxylate Derivatives as Potential Antimicrobial and Anticancer Agents. Int. J. Pharm. Sci. 2014, 6, 341. [Google Scholar]
- Furuya, S.; Ohtaki, T. Pyridopyrimidine Derivatives, Their Production and Use. European Patent EP0608565A1, 7 March 1997. [Google Scholar]
- Broom, A.D.; Shim, J.L.; Bartholomew, D.G.; Anderson, G.L. Synthetic studies leading to various oxopyrido [2, 3-D] pyrimidines. In Abstract of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1975; Volume 170, p. 95. [Google Scholar]
- Özkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial Activity and a SAR Study of Some Novel Benzimidazole Derivatives Bearing Hydrazone Moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef]
- Mamedov, I.; Naghiyev, F.; Maharramov, A.; Uwangue, O.; Farewell, A.; Sunnerhagen, P.; Erdelyi, M. Antibacterial Activity of 2-Amino-3-Cyanopyridine Derivatives. Mendeleev. Commun. 2020, 30, 498–499. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Seshadri, N.; Lavanya, P.; Jonnalagadda, S.B. Synthesis, Antibacterial and Antifungal Activity of Novel Benzothiazole Pyrimidine Derivatives. Arab. J. Chem. 2016, 9, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Reddy, S.; Barathe, P.; Shriram, V.; Anand, U.; Proćków, J.; Kumar, V. Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review. Front. Microbiol. 2021, 12, 747019. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Wabo, G.F.; Ngameni, B.; Mbaveng, A.T.; Metuno, R.; Etoa, F.-X.; Ngadjui, B.T.; Beng, V.P.; Meyer, J.M.; Lall, N. Antimicrobial Activity of the Methanolic Extract, Fractions and Compounds from the Stem Bark of Irvingia Gabonensis (Ixonanthaceae). J. Ethnopharmacol. 2007, 114, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Inform. Suppl. 2011, 31, 100–121. [Google Scholar]
- Asadbegi, S.; Bodaghifard, M.A.; Mobinikhaledi, A. Poly N,N-Dimethylaniline-Formaldehyde Supported on Silica-Coated Magnetic Nanoparticles: A Novel and Retrievable Catalyst for Green Synthesis of 2-Amino-3-Cyanopyridines. Res. Chem. Intermed. 2020, 46, 1629–1643. [Google Scholar] [CrossRef]
- Khaksar, S.; Yaghoobi, M. A Concise and Versatile Synthesis of 2-Amino-3-Cyanopyridine Derivatives in 2,2,2-Trifluoroethanol. J. Fluorine Chem. 2012, 142, 41–44. [Google Scholar] [CrossRef]
- Sarda, S.R.; Kale, J.D.; Wasmatkar, S.K.; Kadam, V.S.; Ingole, P.G.; Jadhav, W.N.; Pawar, R.P. An Efficient Protocol for the Synthesis of 2-Amino-4, 6-Diphenylpyridine-3-Carbonitrile Using Ionic Liquid Ethylammonium Nitrate. Mol. Divers. 2009, 13, 545–549. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N.; Slepokura, K.; Lis, T. Boric Acid as an Efficient and Green Catalyst for the Synthesis of 2-Amino-4, 6-Diarylnicotinonitrile under Microwave Irradiation in Solvent-Free Conditions. Turk. J. Chem. 2019, 43, 464–474. [Google Scholar] [CrossRef]
- Khalifeh, R.; Ghamari, M. A Multicomponent Synthesis of 2-Amino-3-Cyanopyridine Derivatives Catalyzed by Heterogeneous and Recyclable Copper Nanoparticles on Charcoal. J. Braz. Chem. Soc. 2016, 27, 759–768. [Google Scholar] [CrossRef]
- Zengin Kurt, B. Synthesis and Anticholinesterase Activity of Novel Non-Hepatotoxic Naphthyridine-11-Amine Derivatives. Mol. Divers. 2019, 23, 625–638. [Google Scholar] [CrossRef]












| Entry | Amount of Catalyst (g) | Temperature (°C) | Time (Min.) | Yield (%) [a],[b] | |
|---|---|---|---|---|---|
| Absence of a catalyst | 1 | 0 | 80 | 120 | - |
| Influence of the amount of the catalyst | 2 | 0.01 | 80 | 30 | 20 |
| 3 | 0.02 | 80 | 30 | 40 | |
| 4 | 0.03 | 80 | 30 | 60 | |
| 5 | 0.04 | 80 | 30 | 84 | |
| 6 | 0.05 | 80 | 30 | 94 | |
| 7 | 0.06 | 80 | 30 | 94 | |
| 8 | 0.07 | 80 | 30 | 94 | |
| Influence of temperature and reaction time | 9 | 0.05 | 80 | 20 | 65 |
| 10 | 0.05 | 80 | 15 | 53 | |
| 11 | 0.05 | 80 | 40 | 95 | |
| 12 | 0.05 | 40 | 30 | 75 | |
| 13 | 0.05 | 60 | 30 | 85 | |
| 14 | 0.05 | 100 | 30 | 94 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Product [a] | Yield [b] (%) | |
| 1 | H | Ph | H | 5a |  | 94 |
| 2 | CH3 | Ph | H | 5b |  | 85 |
| 3 | CH3O | Ph | H | 5c |  | 84 |
| 4 | Cl | Ph | H | 5d |  | 95 |
| 5 | NO2 | Ph | H | 5e |  | 86 |
| 6 | NO2 | Ph | H | 5f |  | 93 |
| 7 | H | 4-CH3C6H4 | H | 5g |  | 92 |
| 8 | Cl | 4-CH3C6H4 | H | 5h |  | 90 |
| 9 | H | 4-CH3OC6H4 | H | 5i |  | 91 |
| 10 | Cl | 4-CH3OC6H4 | H | 5j |  | 89 |
| 11 | H | -(CH2)4- | 5k |  | 94 | |
| 12 | CH3 | -(CH2)4- | 5l |  | 88 | |
| 13 | Cl | -(CH2)4- | 5m |  | 94 | |
| Entry | 2-Amino-3-Cyanopyridine | Pyrido[2,3-d]pyrimidine [a] | Yield [b] (%) | |
|---|---|---|---|---|
| 1 |  | 6b |  | 74 |
| 2 |  | 6c |  | 71 |
| 3 |  | 6g |  | 81 |
| 4 |  | 6h |  | 79 |
| 5 |  | 6j |  | 71 |
| Cyanopyridine 5a | Cyanopyridine 5b | Pyrimidine 6b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) | MIC (µL/mL) | MBC (µL/mL) | IZD (mm) | MIC (µL/mL) | MBC (µL/mL) | IZD (mm) | MIC (µL/mL) | MBC (µL/mL) | |
| P. aeruginosa (−) | NS | - | - | NS | - | - | NS | - | - |
| S. aureus (+) | NS | - | - | NS | - | - | 21 | 125 | 125 |
| S. epidermidis (+) | NS | - | - | NS | - | - | NS | - | - |
| K. pneumonaie (−) | NS | - | - | NS | - | - | NS | - | - |
| B. subtillis (+) | 18.5 | 64.5 | 64.5 | 17 | 64.5 | 125 | 20.5 | 64.5 | 64.5 |
| E. coli (−) | 13 | 125 | 125 | 12 | 125 | 250 | 12 | 125 | 125 |
| E. feacalis (+) | NS | - | - | NS | - | - | NS | - | - |
| C. albicans | NS | - | - | NS | - | - | NS | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achagar, R.; Elmakssoudi, A.; Thoume, A.; Dakir, M.; Elamrani, A.; Zouheir, Y.; Zahouily, M.; Ait-Touchente, Z.; Jamaleddine, J.; Chehimi, M.M. Nanostructured Na2CaP2O7: A New and Efficient Catalyst for One-Pot Synthesis of 2-Amino-3-Cyanopyridine Derivatives and Evaluation of Their Antibacterial Activity. Appl. Sci. 2022, 12, 5487. https://doi.org/10.3390/app12115487
Achagar R, Elmakssoudi A, Thoume A, Dakir M, Elamrani A, Zouheir Y, Zahouily M, Ait-Touchente Z, Jamaleddine J, Chehimi MM. Nanostructured Na2CaP2O7: A New and Efficient Catalyst for One-Pot Synthesis of 2-Amino-3-Cyanopyridine Derivatives and Evaluation of Their Antibacterial Activity. Applied Sciences. 2022; 12(11):5487. https://doi.org/10.3390/app12115487
Chicago/Turabian StyleAchagar, Redouane, Abdelhakim Elmakssoudi, Abderrahmane Thoume, Mohamed Dakir, Abdelaziz Elamrani, Yassine Zouheir, Mohamed Zahouily, Zouhair Ait-Touchente, Jamal Jamaleddine, and Mohamed M. Chehimi. 2022. "Nanostructured Na2CaP2O7: A New and Efficient Catalyst for One-Pot Synthesis of 2-Amino-3-Cyanopyridine Derivatives and Evaluation of Their Antibacterial Activity" Applied Sciences 12, no. 11: 5487. https://doi.org/10.3390/app12115487