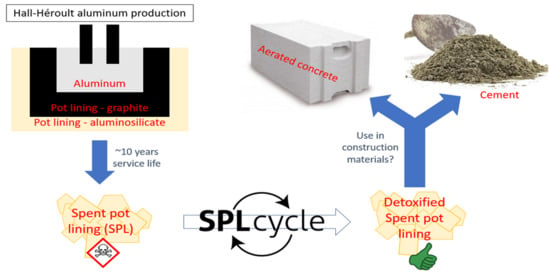
Detoxified Spent Pot Lining from Aluminum Production as (Alumino-)Silicate Source for Composite Cement and AutoClaved Aerated Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the SPL
2.3. The Influence of SPL on Cement Hydration
2.4. Mixing and Testing of Cement Mortars
2.5. Mixing and Testing of Aerated Concrete Blocks
3. Results and Discussion
3.1. Characterization of Spent Pot Lining Aluminosilicates
3.2. Spent Pot Lining Reactivity and Influence on Cement Hydration
3.3. Cement Mortars
3.4. Autoclaved Aerated Concrete
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pong, T.K.; Adrien, R.J.; Besida, J.; O’Donnell, T.A.; Wood, D.G. Spent Potlining—A Hazardous Waste Made Safe. Process. Saf. Environ. Prot. 2000, 78, 204–208. [Google Scholar] [CrossRef]
- Miksa, D.; Homsak, M.; Samec, N. Spent potlining utilisation possibilities. Waste Manag. Res. 2003, 21, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Pingin, V.; Zherdev, A.; Bogdanov, Y.; Pavlov, S.; Somov, V. SPL Recycling and Re-processing. In Proceedings of the Light Metals 2017, Cham, Switzerland; 2017; pp. 571–578. [Google Scholar]
- Holywell, G.; Breault, R. An Overview of Useful Methods to Treat, Recover, or Recycle Spent Potlining. JOM 2013, 65, 1441–1451. [Google Scholar] [CrossRef]
- Kondrat’ev, V.V.; Rzhechitskii, E.P.; Shakhrai, S.G.; Karlina, A.I.; Sysoev, I.A. Recycling of Electrolyzer Spent Carbon-Graphite Lining with Aluminum Fluoride Regeneration. Metallurgist 2016, 60, 571–575. [Google Scholar] [CrossRef] [Green Version]
- International Aluminium Institute. Sustainable Spent Pot Lining Management. 2020. Available online: https://www.world-aluminium.org/publications/ (accessed on 19 April 2021).
- European Commission. EUR-Lex—02000D0532-20150601. In EUR-Lex; European Commission: Brussel, Belgium, 2000. [Google Scholar]
- Tropenauer, B.K.D.; Samec, N.; Golob, J.; Kortnik, J. Sustainable waste-treatment procedure for the spent potlining (SPL) from aluminium production. Mater. Technol. 2019, 53, 277–284. [Google Scholar] [CrossRef]
- Angelopoulos, P.; Dusan, K.; Kosir, M.; Ebin, B.; Bremerstein, I.; Peys, A.; Halilovic, M.; Davris, P.; Taxiarchou, M.; Mladenovic, A. A Novel, Zero-Waste Technology for Spent Pot Lining Recycling. In Proceedings of the 38th International ICSOBA Conference, Online. 16–18 November 2020. [Google Scholar]
- Petrovskiy, A.A.; Nemchinova, N.V.; Tyutrin, A.A.; Korepina, N.A. Use of Leaching Cake from Refractory Lining of Dismantled Electrolysers in Cement Production. In Proceedings of the International Symposium “Engineering and Earth Sciences: Applied and Fundamental Research” dedicated to the 85th anniversary of H.I. Ibragimov (ISEES 2019), August 2019; pp. 358–363. [Google Scholar]
- Global Cement and Concrete Association. Getting the Numbers Right; 2018; Available online: https://gccassociation.org/gnr/ (accessed on 19 April 2021).
- Juenger, M.C.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2019, 7, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Snellings, R.; Li, X.; Avet, F.; Scrivener, K. Rapid, Robust, and Relevant (R3) Reactivity Test for Supplementary Cementitious Materials. ACI Mater. J. 2019, 116, 155–162. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Mitsuda, T.; Sasaki, K.; Ishida, H. Phase evolution during autoclaving process of aerated concrete. J. Am. Ceram. Soc. 1992, 75, 1858–1863. [Google Scholar] [CrossRef]
- Narayanan, N.; Ramamurthy, K. Microstructural investigations on aerated concrete. Cem. Concr. Res. 2000, 30, 457–464. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chang, J.-E.; Lai, Y.-C.; Chou, M.-I.M. A comprehensive study on the production of autoclaved aerated concrete: Effects of silica-lime-cement composition and autoclaving conditions. Constr. Build. Mater. 2017, 153, 622–629. [Google Scholar] [CrossRef]
- Małecki, M.; Kurdowski, W.; Walczak, P. Influence of gypsum and limestone, used as mineral additives, on autoclaved aerated concrete properties. Ce/papers 2018, 2, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S.-Y.; Ogawa, A.; Sato, M. In situ time-resolved X-ray diffraction of tobermorite formation in autoclaved aerated concrete: Influence of silica source reactivity and Al addition. Cem. Concr. Res. 2011, 41, 510–519. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Ni, W.; Cui, W.-H.; Wang, Z.-J.; Zhu, L.-P. Preparation of autoclaved aerated concrete using copper tailings and blast furnace slag. Constr. Build. Mater. 2012, 27, 1–5. [Google Scholar] [CrossRef]
- Isu, N.; Ishida, H.; Mitsuda, T. Influence of quartz particle size on the chemical and mechanical properties of autoclaved aerated concrete (I) tobermorite formation. Cem. Concr. Res. 1995, 25, 243–248. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Shaltout, A.A.; Omar, H.; Abo-El-Enein, S.A. Hydrothermal synthesis and characterization of aluminium and sulfate substituted 1.1nm tobermorites. J. Alloys Compd. 2009, 467, 332–337. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. The influence of sodium salts and gypsum on alite hydration. Cem. Concr. Res. 2015, 75, 53–65. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Müller, A.; Ben Haha, M. Effect of sulfate content on the porosity distribution and resulting performance of composite cements. Constr. Build. Mater. 2018, 186, 912–919. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Factors influencing the sulfate balance in pure phase C3S/C3A systems. Cem. Concr. Res. 2020, 133, 106085. [Google Scholar] [CrossRef]
- Bergmans, J.; Nielsen, P.; Snellings, R.; Broos, K. Recycling of autoclaved aerated concrete in floor screeds: Sulfate leaching reduction by ettringite formation. Constr. Build. Mater. 2016, 111, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C. RILEM TC-238 SCM recommendation on hydration stoppage by solvent exchange for the study of hydrate assemblages. Mater. Struct. 2018, 51, 172. [Google Scholar] [CrossRef]
- Fernández-Jimenez, A.; de la Torre, A.G.; Palomo, A.; López-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Donnay, G.; Schairer, J.F.; Donnay, J.D.H. Nepheline solid solutions. Mineral. Mag. J. Mineral. Soc. 1959, 32, 93–109. [Google Scholar] [CrossRef]
- Snellings, R.; Kamyab, H.; Joseph, S.; Nielsen, P.; Loots, M.; Abeele, L.V.D. Pozzolanic reactivity of size-classified siliceous fly ashes. In Proceedings of the 2nd International Conference of Sustainable Building Materials, Eindhoven, The Netherlands, 12–15 August 2019. [Google Scholar]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Berodier, E.; Bizzozero, J.; Muller, A.C.A. Chapter 9: Mercury Intrusion Porosimetry. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K.S.R., Lothenbach, B., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary Cementitious Materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Hills, C.D.; Sollars, C.J.; Perry, R. A calorimetric and microstructural study of solidified toxic wastes—Part 2: A model for poisoning of OPC hydration. Waste Manag. 1994, 14, 601–612. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, J.; Tian, Z.; Yang, K.; Yao, Z.; Yu, B.; Zhang, L. Comparison of ultrasound-assisted and traditional caustic leaching of spent cathode carbon (SCC) from aluminum electrolysis. Ultrason. Sonochemistry 2018, 40, 21–29. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, Q. Influences of coal fly ash containing ammonium salts on properties of cement paste. J. Environ. Manag. 2019, 249, 109374. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Damidot, D. Managing trace elements in Portland cement—Part I: Interactions between cement paste and heavy metals added during mixing as soluble salts. Cem. Concr. Compos. 2010, 32, 563–570. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN 771-4:2011+A1: Specification for Masonry Units—Part. 4: Autoclaved Aerated Concrete Masonry Units; European Committee for Standardization (CEN): Brussels, Belgium, 2011. [Google Scholar]
- Zafar, M.S.; Javed, U.; Khushnood, R.A.; Nawaz, A.; Zafar, T. Sustainable incorporation of waste granite dust as partial replacement of sand in autoclave aerated concrete. Constr. Build. Mater. 2020, 250, 118878. [Google Scholar] [CrossRef]













| Sample Name | Portland Cement (g) | SPL (g) | Limestone (g) |
|---|---|---|---|
| Reference | 450 | ||
| SPL20 | 360 | 90 | |
| SPL30 | 315 | 135 | |
| SPL40 | 270 | 180 | |
| SPL30-L10 | 270 | 135 | 45 |
| SPL30-L15 | 247.5 | 135 | 67.5 |
| Sample Name | Portland Cement (g) | Lime (g) | SPL (g) | Quartz (g) | Anhydrite (g) | Aluminum Powder (g) |
|---|---|---|---|---|---|---|
| Ref 5 | 30 | 15 | 50 | 5 | 0.1 | |
| SPL 25-5 | 30 | 15 | 12.5 | 37.5 | 5 | 0.1 |
| SPL 50-5 | 30 | 15 | 25 | 25 | 5 | 0.1 |
| SPL 75-5 | 30 | 15 | 37.5 | 12.5 | 5 | 0.1 |
| SPL 100-5 | 30 | 15 | 50 | 5 | 0.1 | |
| Ref 3 | 31 | 16 | 50 | 3 | 0.1 | |
| SPL 25-3 | 31 | 16 | 12.5 | 37.5 | 3 | 0.1 |
| SPL 50-3 | 31 | 16 | 25 | 25 | 3 | 0.1 |
| SPL 75-3 | 31 | 16 | 37.5 | 12.5 | 3 | 0.1 |
| SiO2 | Al2O3 | Na2O | K2O | Fe2O3 | CaO | MgO | TiO2 | |
|---|---|---|---|---|---|---|---|---|
| wt% | 68.0 | 23.8 | 2.8 | 1.8 | 2.4 | 1.4 | 1.3 | 1.1 |
| Phase Name | Phase Formula | Phase Content (wt%) |
|---|---|---|
| Quartz | SiO2 | 17 |
| Mullite | AlxSiO1.5x+2 | 18 |
| Cristobalite | SiO2 | 6 |
| Nepheline | Na3KAl4Si4O16 | 2 |
| Sodalite | Na8Al6Si6O25 | 1 |
| Amorphous | 56 | |
| d10 (µm) | d50 (µm) | d90 (µm) | Specific Surface—BET (m2/g) | Density (kg/m3) | |
|---|---|---|---|---|---|
| Milled SPL | 2.3 | 9.8 | 48.0 | 3.05 | 2697 |
| CEM I 52.5R | 1.2 | 7.1 | 26.0 | 1.55 | 3145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peys, A.; Košir, M.; Snellings, R.; Mladenovič, A.; Horckmans, L. Detoxified Spent Pot Lining from Aluminum Production as (Alumino-)Silicate Source for Composite Cement and AutoClaved Aerated Concrete. Appl. Sci. 2021, 11, 3715. https://doi.org/10.3390/app11083715
Peys A, Košir M, Snellings R, Mladenovič A, Horckmans L. Detoxified Spent Pot Lining from Aluminum Production as (Alumino-)Silicate Source for Composite Cement and AutoClaved Aerated Concrete. Applied Sciences. 2021; 11(8):3715. https://doi.org/10.3390/app11083715
Chicago/Turabian StylePeys, Arne, Mateja Košir, Ruben Snellings, Ana Mladenovič, and Liesbeth Horckmans. 2021. "Detoxified Spent Pot Lining from Aluminum Production as (Alumino-)Silicate Source for Composite Cement and AutoClaved Aerated Concrete" Applied Sciences 11, no. 8: 3715. https://doi.org/10.3390/app11083715