Light-Emitting Biosilica by In Vivo Functionalization of Phaeodactylum tricornutum Diatom Microalgae with Organometallic Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Diatoms Cultures
2.4. In Vivo Functionalization with Organometallic Emitters
2.5. Purification of Doped Frustules
3. Results
3.1. Photostability of Organometallic Complexes in the Culture Medium
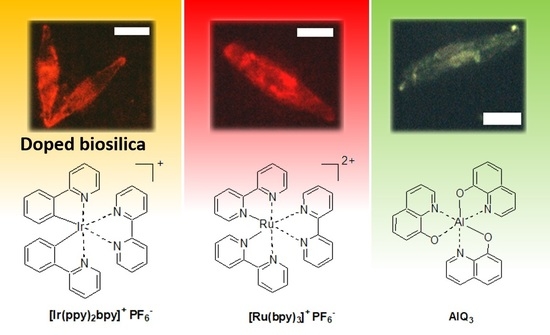
3.2. In Vivo Incorporation of Complexes in Diatoms
3.3. Diatoms Adhesion on ITO Substrates
3.4. Purification and Characterization of Doped Biosilica
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamali, A.A.; Akbari, F.; Moradi Ghorakhlu, M.; de la Guardia, M.; Yari Khosroushahi, A. Applications of Diatoms as Potential Microalgae in Nanobiotechnology. BioImpacts 2012, 2, 83–89. [Google Scholar] [PubMed]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification—Moving Forward. Front. Mar. Sci. 2018, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, M.A.; Dardano, P.; De Stefano, L.; Rea, I.; Coppola, G.; Rendina, I.; Congestri, R.; Antonucci, A.; De Stefano, M.; De Tommasi, E. Optical Properties of Diatom Nanostructured Biosilica in Arachnoidiscus sp: Micro-Optics from Mother Nature. PLoS ONE 2014, 9, e103750. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Landwehr, S.; El Rharbi-Kucki, M.; Sumper, M. Diatoms as living photonic crystals. Appl. Phys. B 2004, 78, 257–260. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom Silica for Biomedical Applications: Recent Progress and Advances. Adv. Healthc. Mater. 2018, 7, e1800552. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant Properties. ChemPlusChem 2015, 80, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Sheppard, V.C.; Ghaemi, S.R.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef] [Green Version]
- Ragni, R.; Cicco, S.R.; Vona, D.; Leone, G.; Farinola, G.M. Biosilica from diatoms microalgae: Smart materials from bio-medicine to photonics. J. Mater. Res. 2017, 32, 279–291. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Leone, G.; De Giglio, E.; Bonifacio, M.A.; Cometa, S.; Fiore, S.; Palumbo, F.; Ragni, R.; Farinola, G.M. In vivo functionalization of diatom biosilica with sodium alendronate as osteoactive material. Mat. Sci. Eng. C 2019, 104, 109897. [Google Scholar] [CrossRef]
- Pan, Z.; Lerch, S.J.L.; Xu, L.; Li, X.; Chuang, Y.-J.; Howe, J.Y.; Mahurin, S.M.; Dai, S.; Hildebrand, M. Electronically transparent graphene replicas of diatoms: A new technique for the investigation of frustule morphology. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.; Vona, D.; Farinola, G.M. Multiple Routes to Smart Nanostructured Materials from Diatom Microalgae: A Chemical Perspective. Adv. Mater. 2018, 30, e1704289. [Google Scholar] [CrossRef]
- Desclés, J.; Vartanian, M.; El Harrak, A.; Quinet, M.; Bremond, N.; Sapriel, G.; Bibette, J.; Lopez, P.J. New tools for labeling silica in living diatoms. New Phytol. 2008, 177, 822–829. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drug. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef]
- Fischer, C.; Adam, M.; Mueller, A.C.; Sperling, E.; Wustmann, M.; van Pée, K.-H.; Kaskel, S.; Brunner, E. Gold Nanoparticle-Decorated Diatom Biosilica: A Favorable Catalyst for the Oxidation of d-Glucose. ACS Omega 2016, 1, 1253–1261. [Google Scholar] [CrossRef] [Green Version]
- Sandhage, K.H.; Dickerson, M.B.; Huseman, P.M.; Caranna, M.A.; Clifton, J.D.; Bull, T.A.; Heibel, T.J.; Overton, W.R.; Schoenwaelder, M.E.A. Novel, bioclastic route to self-assembled, 3D, chemically tailored meso/nanostructures: Shape-preserving reactive conversion of biosilica (diatom) microshells. Adv. Mater. 2002, 14, 429–433. [Google Scholar] [CrossRef]
- Poulsen, N.; Kröger, N.J. Silica Morphogenesis by Alternative Processing of Silaffins in the Diatom Thalassiosira pseudonana. J. Biol. Chem. 2004, 279, 42993–42999. [Google Scholar] [CrossRef] [Green Version]
- Kucki, M.; Fuhrmann-Lieker, T. Staining diatoms with rhodamine dyes: Control of emission colour in photonic biocomposites. J. R. Soc. Interface 2012, 9, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Lo Presti, M.; Ragni, R.; Vona, D.; Leone, G.; Cicco, S.; Farinola, G.M. In vivo doped biosilica from living Thalassiosira weissflogii diatoms with a triethoxysilyl functionalized red emitting fluorophore. MRS Adv. 2018, 3, 1509–1517. [Google Scholar] [CrossRef]
- Ragni, R.; Scotognella, F.; Vona, D.; Moretti, L.; Altamura, E.; Ceccone, G.; Mehn, D.; Cicco, S.R.; Palumbo, F.; Lanzani, G.; et al. Hybrid Photonic Nanostructures by In Vivo Incorporation of an Organic Fluorophore into Diatom Algae. Adv. Funct. Mater. 2018, 28, 1706214. [Google Scholar] [CrossRef]
- Della Rosa, G.; Vona, D.; Aloisi, A.; Ragni, R.; Di Corato, R.; Lo Presti, M.; Cicco, S.R.; Altamura, E.; Taurino, A.; Catalano, M.; et al. Luminescent silica based nanostructures from in vivo Iridium-doped diatoms microalgae. ACS Sustain. Chem. Eng. 2019, 7, 2207–2215. [Google Scholar] [CrossRef]
- Yang, M.; Lin, X.; Liu, X.; Zhang, J.; Ge, F. Genome Annotation of a Model Diatom Phaeodactylum tricornutum Using an Integrated Proteogenomic Pipeline. Mol. Plant 2018, 11, 1292–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brütting, W.; Frischeisen, J.; Schmidt, T.D.; Scholz, B.J.; Mayr, C. Device efficiency of organic light-emitting diodes: Progress by improved light outcoupling. IPSS 2013, 210, 44–65. [Google Scholar] [CrossRef]
- D’Andrade, B. Phosphorescent OLEDs for solid-state lighting. In Organic Light-Emitting Diodes (OLEDs); Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2013; pp. 143–169. [Google Scholar]
- Ragni, R.; Maiorano, V.; Pugliese, M.; Maggiore, A.; Orselli, E.; Babudri, F.; Gigli, G.; De Cola, L.; Farinola, G.M. A highly fluorinated iridium complex as a blue-green emitting component for white electroluminescence. Synth. Met. 2017, 227, 148–155. [Google Scholar] [CrossRef]
- Mao, H.-T.; Li, G.-F.; Shan, G.-G.; Wang, X.-L.; Su, Z.-M. Recent progress in phosphorescent Ir(III) complexes for nondoped organic light-emitting diodes. Coord. Chem. Rev. 2020, 413, 213283. [Google Scholar] [CrossRef]
- Collins, A.M.; Olof, S.M.; Mitchelsa, J.M.; Mann, S. Facile preparation and processing of aqueous dispersions of tris(8-hydroxyquinoline) aluminium(III) photoluminescent nanoparticles. J. Mater. Chem. 2009, 19, 3950–3954. [Google Scholar] [CrossRef]
- Pan, H.-C.; Liang, F.-P.; Mao, C.-J.; Zhu, J.-J.; Chen, H.-Y. Highly Luminescent Zinc(II)−Bis(8-hydroxyquinoline) Complex Nanorods: Sonochemical Synthesis, Characterizations, and Protein Sensing. J. Phys. Chem. B 2007, 111, 5767–5772. [Google Scholar] [CrossRef]
- Carreño, A.; Páez-Hernández, D.; Zúñiga, C.; Ramírez-Osorio, A.; Nevermann, J.; Rivera-Zaldívar, M.M.; Otero, C.; Fuentes, J.A. Prototypical cis-ruthenium(II) complexes present differential fluorescent staining in walled-cell models (yeasts). Chem. Pap. 2019, 73, 1629–1637. [Google Scholar] [CrossRef]
- Schneider, G.E.; Bolink, H.J.; Constable, E.C.; Ertl, C.D.; Housecroft, C.E.; Pertegàs, A.; Zampese, J.A.; Kanitz, A.; Kessler, F.B.; Meier, S. Chloride ion impact on materials for light-emitting electrochemical cells. Dalton Trans. 2014, 43, 1961–1964. [Google Scholar] [CrossRef] [Green Version]
- Vrieling, E.C.; Gieskes, W.W.C. Silicon Deposition in diatoms: Control by the pH inside the silicon deposition vesicle. J. Phycol. 1999, 35, 548–559. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; Cioffi, C.T.; Farinola, G.M.; Naso, F.; Ragni, R. A straightforward methodology for the introduction of aryl and vinyl substituents in the 5 or 7 position of 8-hydroxyquinoline. Synthesis 2006, 8, 1325–1332. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef] [Green Version]
- De Tommasi, E. Light Manipulation by Single Cells: The Case of Diatoms. J. Spectrosc. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- McNeil, L.E.; Grimsditch, M.; French, R.H. Vibrational spectroscopy of aluminum nitride. J. Am. Ceram. Soc. 1993, 76, 1132–1136. [Google Scholar] [CrossRef]
- Sergeeva, A.V.; Zhitova, E.S.; Nuzhdaev, A.A.; Zolotarev, A.A.; Bocharov, V.N.; Ismagilova, R.M. Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18. Minerals 2020, 10, 781. [Google Scholar] [CrossRef]
- Martínez-Ramírez, S.; Gutierrez-Contreras, R.; Husillos-Rodriguez, N.; Fernandez-Carrasco, L. In-situ reaction of the very early hydration of C3A-gypsum-sucrose system by Micro-Raman spectroscopy. Cem. Concr. Compos. 2016, 73, 251–256. [Google Scholar] [CrossRef]
- Doorn, S.K.; Hupp, J.T. Preresonance Raman studies of metal-to-ligand charge transfer in (NH3)4Ru(2,2’-bipyridine)2+. In situ bond length changes, force constants, and reorganization energies. J. Am. Chem. Soc. 1989, 11, 4704–4712. [Google Scholar] [CrossRef]
- Lai, S.H.; Ling, J.W.; Huang, Y.M.; Huang, M.J.; Cheng, C.H.; Chen, I.C. Characterization of Ir(ppy)3 and [Ir(ppy)2 bpy]+ by infrared, Raman spectra and surface-enhanced Raman scattering. J. Raman Spectrosc. 2011, 42, 332–338. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vona, D.; Ragni, R.; Altamura, E.; Albanese, P.; Giangregorio, M.M.; Cicco, S.R.; Farinola, G.M. Light-Emitting Biosilica by In Vivo Functionalization of Phaeodactylum tricornutum Diatom Microalgae with Organometallic Complexes. Appl. Sci. 2021, 11, 3327. https://doi.org/10.3390/app11083327
Vona D, Ragni R, Altamura E, Albanese P, Giangregorio MM, Cicco SR, Farinola GM. Light-Emitting Biosilica by In Vivo Functionalization of Phaeodactylum tricornutum Diatom Microalgae with Organometallic Complexes. Applied Sciences. 2021; 11(8):3327. https://doi.org/10.3390/app11083327
Chicago/Turabian StyleVona, Danilo, Roberta Ragni, Emiliano Altamura, Paola Albanese, Maria Michela Giangregorio, Stefania Roberta Cicco, and Gianluca Maria Farinola. 2021. "Light-Emitting Biosilica by In Vivo Functionalization of Phaeodactylum tricornutum Diatom Microalgae with Organometallic Complexes" Applied Sciences 11, no. 8: 3327. https://doi.org/10.3390/app11083327