Fast and Low-Cost Synthesis of MoS2 Nanostructures on Paper Substrates for Near-Infrared Photodetectors
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
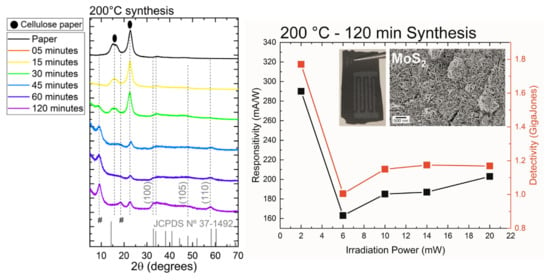
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torrisi, F.; Carey, T. Graphene, Related Two-Dimensional Crystals and Hybrid Systems for Printed and Wearable Electronics. Nano Today 2018, 23, 73–96. [Google Scholar] [CrossRef]
- Bhatt, K.; Kumar, S.; Tripathi, C.C. High-Performance Ultra-Low Leakage Current Graphene-Based Screen-Printed Field-Effect Transistor on Paper Substrate. Pramana J. Phys. 2020, 94, 2–5. [Google Scholar] [CrossRef]
- Song, H.; Liu, J.; Lu, H.; Chen, C.; Ba, L. High Sensitive Gas Sensor Based on Vertical Graphene Field Effect Transistor. Nanotechnology 2020, 31, 165503. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Di, J.; Zhu, W.; Li, H. Hexagonal Boron Nitride Adsorbent: Synthesis, Performance Tailoring and Applications. J. Energy Chem. 2020, 40, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Qiu, W.; Liu, J.; Yu, L.; Gao, S.; Yao, H.; Chen, Y.; Shi, J. Silicene: Wet-Chemical Exfoliation Synthesis and Biodegradable Tumor Nanomedicine. Adv. Mater. 2019, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, B.; Liu, X.; Wang, L.; Zhang, Z.; Mannix, A.J.; Fisher, B.L.; Yakobson, B.I.; Hersam, M.C.; Guisinger, N.P. Borophene Synthesis on Au(111). ACS Nano 2019, 13, 3816–3822. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zeng, G.; Huang, D.; Qin, L.; Zhang, M.; Cheng, M.; Liu, X.; Yi, H.; Zhou, C.; et al. Black Phosphorus, a Rising Star 2D Nanomaterial in the Post-Graphene Era: Synthesis, Properties, Modifications, and Photocatalysis Applications. Small 2019, 15, 1–30. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Sriram, P.; Manikandan, A.; Chuang, F.C.; Chueh, Y.L. Hybridizing Plasmonic Materials with 2D-Transition Metal Dichalcogenides toward Functional Applications. Small 2020. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Gao, Y.; Luo, P.; Su, J.; Han, W.; Liu, K.; Li, H.; Zhai, T. 2D Metal Chalcogenides for IR Photodetection. Small 2019, 15. [Google Scholar] [CrossRef]
- Wypych, F.; Schoellhorn, R. 1T-MoS2, A New Metallic Modification of Molybdenum Disulfide. ChemInform 1992, 24, 1386–1388. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, Y.; Liu, J.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Microwave-Assisted 1T to 2H Phase Reversion of MoS2 in Solution: A Fast Route to Processable Dispersions of 2H-MoS2 Nanosheets and Nanocomposites. Nanotechnology 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS 2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Munkhbayar, G.; Palleschi, S.; Perrozzi, F.; Nardone, M.; Davaasambuu, J.; Ottaviano, L. A Study of Exfoliated Molybdenum Disulfide (MoS2) Based on Raman and Photoluminescence Spectroscopy. Solid State Phenom. 2018, 271, 40–46. [Google Scholar] [CrossRef]
- Neville, R.A.; Evans, B.L. The Band Edge Excitons in 2H-MoS2. Phys. Status Solidi 1976, 73, 597–606. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Zheng, W.; Liu, X.; Zhang, J.; Kumar, M. MoS2-Based Nanomaterials for Room-Temperature Gas Sensors. Adv. Mater. Technol. 2020, 1–28. [Google Scholar] [CrossRef]
- Joyner, J.; Oliveira, E.F.; Yamaguchi, H.; Kato, K.; Vinod, S.; Galvao, D.S.; Salpekar, D.; Roy, S.; Martinez, U.; Tiwary, C.S.; et al. Graphene Supported MoS2 Structures with High Defect Density for an Efficient HER Electrocatalysts. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Singh, E.; Singh, P.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Flexible Molybdenum Disulfide (MoS 2) Atomic Layers for Wearable Electronics and Optoelectronics. ACS Appl. Mater. Interfaces 2019, 11, 11061–11105. [Google Scholar] [CrossRef]
- Zhang, T.; Feng, Y.; Zhang, J.; He, C.; Itis, D.; Song, J. Ultrahigh-Rate Sodium-ion Battery Anode Enable by Vertically Aligned (1T-2H MoS2)/CoS2 Hetero-nanosheets. Mater. Today Nano 2020. [Google Scholar] [CrossRef]
- Liao, F.; Deng, J.; Chen, X.; Wang, Y.; Zhang, X.; Liu, J.; Zhu, H.; Chen, L.; Sun, Q.; Hu, W.; et al. A Dual-Gate MoS2 Photodetector Based on Interface Coupling Effect. Small 2020, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Huo, N.; Konstantatos, G. Recent Progress and Future Prospects of 2D-Based Photodetectors. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; Cauley, J.; Colemanm, J.N. Large-scale production of size-controlled MoS2 nanosheets by shear exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Ottaviano, L.; Palleschi, S.; Perozzi, F.; D’Olimpio, G.; Priante, F.; Donarelli, M.; Benassi, P.; Nardone, M.; Gonchigsuren, M.; Gombosuren, M.; et al. Mechanical Exfoliation and Layer Number Identification of MoS2 Revisited. 2D Mater. 2017, 4, 045013. [Google Scholar] [CrossRef]
- Liu, H.F.; Wong, S.L.; Chi, D.Z. CVD Growth of MoS2-Based Two-Dimensional Materials. Chem. Vap. Depos. 2015, 21, 241–259. [Google Scholar] [CrossRef]
- Tang, G.; Sun, J.; Wei, C.; Wu, K.; Ji, X.; Liu, S.; Tang, H.; Li, C. Synthesis and Characterization of Flowerlike MoS 2 Nanostructures through CTAB-Assisted Hydrothermal Process. Mater. Lett. 2012, 86, 9–12. [Google Scholar] [CrossRef]
- Lee, C.M.; Park, G.C.; Lee, S.M.; Choi, J.H.; Jeong, S.H.; Seo, T.Y.; Jung, S.B.; Lim, J.H.; Joo, J. Effects of Precursor Concentration on Morphology of MoS2 Nanosheets by Hydrothermal Synthesis. J. Nanosci. Nanotechnol. 2016, 16, 11548–11551. [Google Scholar] [CrossRef]
- Sahatiya, P.; Jones, S.S.; Badhulika, S. Direct, Large Area Growth of Few-Layered {MoS}2 Nanostructures on Various Flexible Substrates: Growth Kinetics and Its Effect on Photodetection Studies. Flex. Print. Electron. 2018, 3, 15002. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Gao, M.R.; Chan, M.K.Y.; Sun, Y. Edge-Terminated Molybdenum Disulfide with a 9.4-Å Interlayer Spacing for Electrochemical Hydrogen Production. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Hu, X.; Sun, Q.; Hao, Y.; Wu, H.; Zhang, D.; Bai, J.; Liu, E.; Fan, J.; Hou, X. Hydrothermal Synthesis of MoS2 Nanosheets Films: Microstructure and Formation Mechanism Research. Mater. Lett. 2016, 166, 121–124. [Google Scholar] [CrossRef]
- Gomathi, P.T.; Sahatiya, P.; Badhulika, S. Large-Area, Flexible Broadband Photodetector Based on ZnS–MoS2 Hybrid on Paper Substrate. Adv. Funct. Mater. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Sahatiya, P.; Kadu, A.; Gupta, H.; Gomathi, T.P.; Badhulika, S. Flexible, Disposable Cellulose-Paper-Based MoS2/Cu2S Hybrid for Wireless Environmental Monitoring and Multifunctional Sensing of Chemical Stimuli. ACS Appl. Mater. Interfaces 2018, 10, 9048–9059. [Google Scholar] [CrossRef]
- Sahatiya, P.; Badhulika, S. Wireless, Smart, Human Motion Monitoring Using Solution Processed Fabrication of Graphene–MoS2 Transistors on Paper. Adv. Electron. Mater. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Grey, P.; Gaspar, D.; Cunha, I.; Barras, R.; Carvalho, J.T.; Ribas, J.R.; Fortunato, E.; Martins, R.; Pereira, L. Handwritten Oxide Electronics on Paper. Adv. Mater. Technol. 2017, 2, 2–8. [Google Scholar] [CrossRef]
- Martins, R.; Nathan, A.; Barros, R.; Pereira, L.; Barquinha, P.; Correia, N.; Costa, R.; Ahnood, A.; Ferreira, I.; Fortunato, E. Complementary Metal Oxide Semiconductor Technology with and on Paper. Adv. Mater. 2011, 23, 4491–4496. [Google Scholar] [CrossRef]
- Martins, R.; Ferreira, I.; Fortunato, E. Electronics with and on Paper. Phys. Status Solidi Rapid Res. Lett. 2011, 5, 332–335. [Google Scholar] [CrossRef]
- Barras, R.; Cunha, I.; Gaspar, D.; Fortunato, E.; Martins, R.; Pereira, L. Printable Cellulose-Based Electroconductive Composites for Sensing Elements in Paper Electronics. Flex. Print. Electron. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Smolander, M. Paper as Active Layer in Inkjet-Printed Capacitive Humidity Sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef] [Green Version]
- Águas, H.; Mateus, T.; Vicente, A.; Gaspar, D.; Mendes, M.J.; Schmidt, W.A.; Pereira, L.; Fortunato, E.; Martins, R. Thin Film Silicon Photovoltaic Cells on Paper for Flexible Indoor Applications. Adv. Funct. Mater. 2015, 25, 3592–3598. [Google Scholar] [CrossRef]
- Cunha, I.; Barras, R.; Grey, P.; Gaspar, D.; Fortunato, E.; Martins, R.; Pereira, L. Reusable Cellulose-Based Hydrogel Sticker Film Applied as Gate Dielectric in Paper Electrolyte-Gated Transistors. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Purandare, S.; Gomez, E.F.; Steckl, A.J. High Brightness Phosphorescent Organic Light Emitting Diodes on Transparent and Flexible Cellulose Films. Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Freire, T.; Barranger, A.; Vieira, J.; Matias, M.; Pereira, S.; Pimentel, A.; Cordeiro, N.J.A.; Fortunato, E.; Martins, R. TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Appl. Sci. 2020, 10, 1200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Guo, M.; Wang, L.; Ding, Y.; Zhang, Z.; Tang, Y.; Liu, C.; Luo, S. 1T-MoS2 Nanosheets Confined among TiO2 Nanotube Arrays for High Performance Supercapacitor. Chem. Eng. J. 2019, 366, 163–171. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Han, Y.; Li, J.; Yang, L.; Benamara, M.; Chen, L.; Zhu, H. Two-Dimensional Water-Coupled Metallic MoS2 with Nanochannels for Ultrafast Supercapacitors. Nano Lett. 2017, 17, 1825–1832. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Chen, W.; Chang, K.; Ma, L.; Huang, G.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2–graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A. 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, D.; Wang, H.; Hu, Y.; Guo, S.; Yuan, H.; Li, C. 2D monolayer MoS2-carbon interoverlapped superstructure: Engineering ideal atomic interface for lithium ion storage. Adv. Mater. 2015, 24, 3687–3695. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Song, L. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: Applications for visible-light-driven photocatalytic hydrogen evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef]
- Shao, J.; Qu, Q.; Wan, Z.; Gao, T.; Zuo, Z.; Zheng, H. From dispersed microspheres to interconnected nanospheres: Carbon-sandwiched monolayered MoS2 as high-performance anode of Li-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 22927–22934. [Google Scholar]
- Wu, M.; Zhan, J.; Wu, K.; Li, Z.; Wang, L.; Geng, B.; Pan, D. Metallic 1T MoS 2 nanosheet arrays verticaly grown on activated carbon fiber cloth for enhanced Li-ion storage performance. J. Mater. Chem. A 2017, 5, 14061–14069. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and strage. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Mohan, M.; Timung, R.; Deshavath, N.N.; Banerjee, T.; Goud, V.V.; Dasu, V.V. Optimization and Hydrolysis of Cellulose under Subcritical Water Treatment for the Production of Total Reducing Sugars. RSC Adv. 2015, 5, 103265–103275. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Li, J.; Pan, Z.; Ma, H.; Jiang, Y.; Tian, Z. Facile hydrothermal synthesis of MoS 22 nano-sheets with controllable structures and enhanced catalytic performance for anthracene hydrogeneration. RSC Adv. 2016, 6, 71534–71542. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khanuja, M.; Islam, S.S. Hydrothermal Synthesis of MoS2 Nanosheets for Multiple Wavelength Optical Sensing Applications. Sens. Actuators A Phys. 2018, 277, 190–198. [Google Scholar] [CrossRef]
- Jing, W.; Ding, N.; Li, L.; Jiang, F.; Xiong, X.; Liu, N.; Zhai, T.; Gao, Y. Ag Nanoparticles Modified Large Area Monolayer MoS_2 Phototransistors with High Responsivity. Opt. Express 2017, 25. [Google Scholar] [CrossRef]
- Wang, W.; Klots, A.; Prasai, D.; Yang, Y.; Bolotin, K.I.; Valentine, J. Hot Electron-Based Near-Infrared Photodetection Using Bilayer MoS 2. Nano Lett. 2015, 15, 7440–7444. [Google Scholar] [CrossRef]
- Hong, T.; Chamlagain, B.; Hu, S.; Weiss, S.M.; Zhou, Z.; Xu, Y.Q. Plasmonic Hot Electron Induced Photocurrent Response at MoS 2 –Metal Junctions. ACS Nano 2015, 9, 5357–5363. [Google Scholar] [CrossRef]
- Liu, B.; Gutha, R.R.; Kattel, B.; Alamri, M.; Gong, M.; Sadeghi, S.M.; Chan, W.L.; Wu, J.Z. Using Silver Nanoparticles-Embedded Silica Metafilms as Substrates to Enhance the Performance of Perovskite Photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 32301–32309. [Google Scholar] [CrossRef]
- Wang, Y.; Fullon, R.; Acerce, M.; Petoukhoff, C.E.; Yang, J.; Chen, C.; Du, S.; Lai, S.K.; Lau, S.P.; Voiry, D.; et al. Solution-Processed MoS2/Organolead Trihalide Perovskite Photodetectors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Wang, H.; Lim, J.W.; Quan, L.N.; Chung, K.; Jang, Y.J.; Ma, Y.; Kim, D.H. Perovskite–Gold Nanorod Hybrid Photodetector with High Responsivity and Low Driving Voltage. Adv. Opt. Mater. 2018, 6. [Google Scholar] [CrossRef]
- Yu, W.J.; Liu, Y.; Zhou, H.; Yin, A.; Li, Z.; Huang, Y.; Duan, X. Highly Efficient Gate-Tunable Photocurrent Generation in Vertical Heterostructures of Layered Materials. Nat. Nanotechnol. 2013, 8, 952–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, N.J.A.; Gaspar, C.; Oliveira, M.J.d.; Nunes, D.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R.; Laureto, E.; Lourenço, S.A. Fast and Low-Cost Synthesis of MoS2 Nanostructures on Paper Substrates for Near-Infrared Photodetectors. Appl. Sci. 2021, 11, 1234. https://doi.org/10.3390/app11031234
Cordeiro NJA, Gaspar C, Oliveira MJd, Nunes D, Barquinha P, Pereira L, Fortunato E, Martins R, Laureto E, Lourenço SA. Fast and Low-Cost Synthesis of MoS2 Nanostructures on Paper Substrates for Near-Infrared Photodetectors. Applied Sciences. 2021; 11(3):1234. https://doi.org/10.3390/app11031234
Chicago/Turabian StyleCordeiro, Neusmar J. A., Cristina Gaspar, Maria J. de Oliveira, Daniela Nunes, Pedro Barquinha, Luís Pereira, Elvira Fortunato, Rodrigo Martins, Edson Laureto, and Sidney A. Lourenço. 2021. "Fast and Low-Cost Synthesis of MoS2 Nanostructures on Paper Substrates for Near-Infrared Photodetectors" Applied Sciences 11, no. 3: 1234. https://doi.org/10.3390/app11031234