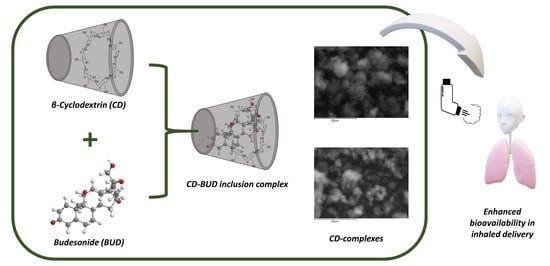
β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Inclusion Complexes
2.3. Inclusion Complexes Characterization
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. Nuclear Magnetic Resonance (NMR)
2.3.3. Wide-Angle X-ray Scattering (XRD)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. High-Pressure Liquid Chromatography (HPLC), Quantitative Analysis and Drug Loading
2.3.8. Drug-Loading Percentage
2.3.9. Phase-Solubility Studies
2.3.10. In Vitro Dissolution Studies
3. Results and Discussion
3.1. The Effect of Solvent Ratio in Budesonide Cyclodextrin Inclusion Complexes
3.2. Budesonide Inclusion in Cyclodextrin Complexes
3.3. In Vitro Drug Release Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ye, J.; Yao, P.; Shi, X.; Yu, X. A systematic literature review and meta-analysis on the impact of COPD on atrial fibrillation patient outcome. Hear. Lung 2022, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Villegas, C.; Paz-Zulueta, M.; Herrero-Montes, M.; Parás-Bravo, P.; Madrazo Pérez, M. Cost analysis of chronic obstructive pulmonary disease (COPD): A systematic review. Health Econ. Rev. 2021, 11, 31. [Google Scholar] [CrossRef]
- Zheng, J.; Baldi, S.; Zhao, L.; Li, H.; Lee, K.H.; Singh, D.; Papi, A.; Grapin, F.; Guasconi, A.; Georges, G. Efficacy and safety of single-inhaler extrafine triple therapy versus inhaled corticosteroid plus long-acting beta2 agonist in eastern Asian patients with COPD: The TRIVERSYTI randomised controlled trial. Respir. Res. 2021, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Rutishauser, J.; Ulrik, C.S.; Leuppi, J.D.; Pedersen, L.; Mueller, B.; Eklöf, J.; Biering-Sørensen, T.; Gottlieb, V.; Armbruster, K.; et al. Effect of different corticosteroid regimes for hospitalised patients with exacerbated COPD: Pooled analysis of individual participant data from the REDUCE and CORTICO-COP trials. Respir. Res. 2021, 22, 155. [Google Scholar] [CrossRef] [PubMed]
- Koarai, A.; Yamada, M.; Ichikawa, T.; Fujino, N.; Kawayama, T.; Sugiura, H. Triple versus LAMA/LABA combination therapy for patients with COPD: A systematic review and meta-analysis. Respir. Res. 2021, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Odonnell, S.; Omorain, C.A. Therapeutic benefits of budesonide in gastroenterology. Ther. Adv. Chronic Dis. 2010, 1, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ansari, M.M.; Kumar, A.; Bishnoi, M.; Raza, S.S.; Khan, R. Aminocellulose-grafted polycaprolactone-coated core-shell nanoparticles alleviate the severity of ulcerative colitis: A novel adjuvant therapeutic approach. Biomater. Sci. 2021, 9, 5868–5883. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of Poly ( vinyl alcohol ) on Nanoencapsulation of Budesonide in Chitosan Nanoparticles via Ionic Gelation and Its Improved Bioavailability. Polymers 2020, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Costa, T.G.D.; De Lima, R.; Hedtrich, S.; Fraceto, L.F.; De Araujo, D.R. Hydrogels Containing Budesonide-Loaded Nanoparticles to Facilitate Percutaneous Absorption for Atopic Dermatitis Treatment Applications. ACS Appl. Polym. Mater. 2021, 3, 4436–4449. [Google Scholar] [CrossRef]
- Wang, S.; Wannasarit, S.; Figueiredo, P.; Molinaro, G.; Ding, Y.; Correia, A.; Casettari, L.; Wiwattanapatapee, R.; Hirvonen, J.; Liu, D.; et al. Intracellular Delivery of Budesonide and Polydopamine Co-Loaded in Endosomolytic Poly(butyl methacrylate-co-methacrylic acid) Grafted Acetalated Dextran for Macrophage Phenotype Switch from M1 to M2. Adv. Ther. 2021, 4, 2000058. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Rakmai, J. Inclusion complex formation of cyclodextrin with its guest and their applications. Biol. Eng. Med. 2017, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-drug inclusion complexes: In Vivo and In Vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Papadopoulos, N.D.; Karayianni, H.S.; Tsakiridis, P.E.; Perraki, M.; Hristoforou, E. Cyclodextrin inclusion complexes as novel MOCVD precursors for potential cobalt oxide deposition. Appl. Organomet. Chem. 2010, 24, 112–121. [Google Scholar] [CrossRef]
- Shaikh, J.; Deshmane, S.V.; Purohit, R.N.; Biyani, K.R. Behavioural study of cyclodextrin inclusion complex on enhancement of solubility of aceclofenac. Indian Drugs 2015, 52, 19–23. [Google Scholar] [CrossRef]
- Jollet, V.; Chambon, F.; Rataboul, F.; Cabiac, A.; Pinel, C.; Guillon, E.; Essayem, N. Non-catalyzed and Pt/γ-Al2O3-catalyzed hydrothermal cellulose dissolution–conversion: Influence of the reaction parameters and analysis of the unreacted cellulose. Green Chem. 2009, 11, 2052–2060. [Google Scholar] [CrossRef]
- Leng, D.; Thanki, K.; Foged, C.; Yang, M. Formulating Inhalable Dry Powders Using Two-Fluid and Three-Fluid Nozzle Spray Drying. Pharm. Res. 2018, 35, 247. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Maheriya, P.M. Cyclodextrin: A promising candidate in enhancing oral bioavailability of poorly water soluble drugs. MOJ Bioequiv. Bioavailab. (MOJBB) 2017, 3, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Ono, N.; Hirayama, F.; Arima, H.; Uekama, K. Analysis of the phase solubility diagram of a phenacetin/competitor/β-cyclodextrin ternary system, involving competitive inclusion complexation. Chem. Pharm. Bull. 2001, 49, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Bayrakc, M.; Ertul, Ş.; Yilmaz, M. Phase solubility studies of poorly soluble drug molecules by using O-phosphorylated calixarenes as drug-solubilizing agents. J. Chem. Eng. Data 2012, 57, 233–239. [Google Scholar] [CrossRef]
- Boonyarattanakalin, K.; Viernstein, H.; Wolschann, P.; Lawtrakul, L. Influence of ethanol as a Co-Solvent in cyclodextrin inclusion complexation: A Molecular Dynamics Study. Sci. Pharm. 2015, 83, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Liu, B.; Liu, H. Characterization of hydroxypropyl-β-cyclodextrins with different substitution patterns via FTIR, GC-MS, and TG-DTA. Carbohydr. Polym. 2015, 118, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.J.; Pawar, A.P.; Purohit, R.N. Development of Budesonide Loaded Biopolymer Based Dry Powder Inhaler: Optimization, In Vitro Deposition, and Cytotoxicity Study. J. Pharm. 2014, 2014, 795271. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Silva, A.L.N.; Viana, L.G.F.C.; Silva, M.G.; Lavor, É.M.; Oliveira-Júnior, R.G.; Alencar-Filho, E.B.; Lima, R.S.; Mendes, R.L.; Rolim, L.A.; et al. β-Cyclodextrin complex improves the bioavailability and antitumor potential of cirsiliol, a flavone isolated from Leonotis nepetifolia (Lamiaceae). Heliyon 2019, 5, 1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Sarbu, I.; Popa, V.T.; Munteanu, C.; Mircioiu, C.; Ozon, E.A.; Nitulescu, G.M.; Mitu, M.A. Formulation of chewable tablets containing carbamazepine-β-cyclodextrin inclusion complex and f-melt disintegration excipient. The mathematical modeling of the release kinetics of carbamazepine. Pharmaceutics 2021, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Weigmann, B.; Collnot, E.M.; Khan, S.A.; Windbergs, M.; Lehr, C.M. Budesonide loaded PLGA nanoparticles for targeting the inflamed intestinal mucosa—Pharmaceutical characterization and fluorescence imaging. Pharm. Res. 2016, 33, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, S.; Zimmermann, A.; Totóli, E.G.; Salgado, H.R.N. FTIR Spectrophotometry as a Green Tool for Quantitative Analysis of Drugs: Practical Application to Amoxicillin. J. Chem. 2018, 2018, 3920810. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Burapapadh, K. Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization. Asian J. Pharm. Sci. 2015, 10, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Mezzena, M.; Scalia, S.; Young, P.M.; Traini, D. Solid lipid budesonide microparticles for controlled release inhalation therapy. AAPS J. 2009, 11, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikowitz, K.; Pintye-Hódi, K.; Regdon, G. Study of the recrystallization in coated pellets—Effect of coating on API crystallinity. Eur. J. Pharm. Sci. 2013, 48, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, R.; Ficarra, P.; Di Bella, M.R.; Raneri, D.; Tommasini, S.; Calabrò, M.L.; Gamberini, M.C.; Rustichelli, C. Study of β-blockers/β-cyclodextrins inclusion complex by NMR, DSC, X-ray and SEM investigation. J. Pharm. Biomed. Anal. 2000, 23, 33–40. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Teja, S.B.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, A.K. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2014, 4, 70–94. [Google Scholar]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin-drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Rojanarata, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Sila-On, W.; Opanasopit, P. Improvement of drug loading onto ion exchange resin by cyclodextrin inclusion complex. Drug Dev. Ind. Pharm. 2013, 39, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Karavas, E.; Georgarakis, E.; Sigalas, M.P.; Avgoustakis, K.; Bikiaris, D. Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug-polymer interactions. Eur. J. Pharm. Biopharm. 2007, 66, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.Z.; Bikiaris, D.; Kanaze, F.I.; Karavas, E.; Stergiou, A.; Georgarakis, E. Tailoring the release rates of fluconazole using solid dispersions in polymer blends. Drug Dev. Ind. Pharm. 2008, 34, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Ainali, N.M.; Xanthopoulou, E.; Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Microencapsulation of fluticasone propionate and salmeterol xinafoate in modified chitosan microparticles for release optimization. Molecules 2020, 25, 3888. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, N.Ü.; Mone, M.; Giannakopoulou, S.; Er, S.; Pavlidou, E.; Karavas, E.; Bikiaris, D.N. Two different approaches for oral administration of voriconazole loaded formulations: Electrospun fibers versus β-cyclodextrin complexes. Int. J. Mol. Sci. 2016, 17, 282. [Google Scholar] [CrossRef] [PubMed]














| Sample | % of Crystallinity |
|---|---|
| Budesonide | 70.4 |
| Cyclodextrin | 72.2 |
| CD–BUD-10_50-50 | 68.6 |
| CD–BUD-10_60-40 | 64.8 |
| CD–BUD-10_70-30 | 59.1 |
| CD–BUD-10_80-20 | 45.4 |
| CD–BUD-10-90-10 | 68.4 |
| Sample | Degree of Crystallinity (%) |
|---|---|
| CD–BUD-5% | 43.9 |
| CD–BUD-10% | 45.4 |
| CD–BUD-20% | 53.6 |
| CD–BUD-30% | 56.6 |
| Sample | Drug Loading (%) |
|---|---|
| CD–BUD-10_50-50 | 8.8 |
| CD–BUD-10_60-40 | 8.9 |
| CD–BUD-10_70-30 | 8.8 |
| CD–BUD-10_80-20 | 9.1 |
| CD–BUD-10-90-10 | 7.8 |
| Sample | Drug Loading |
|---|---|
| CD–BUD-5% | 2.1 |
| CD–BUD-10% | 8.8 |
| CD–BUD-20% | 13.7 |
| CD–BUD-30% | 19.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidou, G.; Papageorgiou, G.Z.; Bikiaris, D.N. β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment. Appl. Sci. 2021, 11, 12085. https://doi.org/10.3390/app112412085
Michailidou G, Papageorgiou GZ, Bikiaris DN. β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment. Applied Sciences. 2021; 11(24):12085. https://doi.org/10.3390/app112412085
Chicago/Turabian StyleMichailidou, Georgia, George Z. Papageorgiou, and Dimitrios N. Bikiaris. 2021. "β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment" Applied Sciences 11, no. 24: 12085. https://doi.org/10.3390/app112412085