Mathematical Model for Scaling up Bioprocesses Using Experiment Design Combined with Buckingham Pi Theorem
Abstract
:Featured Application
Abstract
1. Introduction
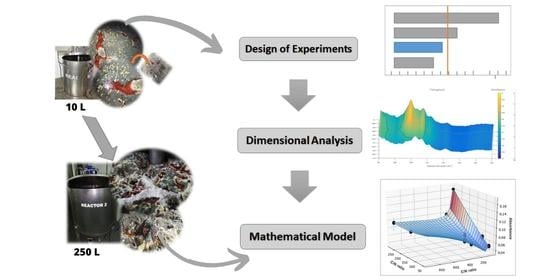
2. Materials and Methods
2.1. Bioprocess
2.2. Mathematical Model
- C: Carbon Source Concentration (kg/m3);
- N: Nitrogen Source Concentration (kg/m3);
- K: Potassium Source Concentration (kg/m3);
- T: Trace Source Concentration (kg/m3);
- A: Agitation speed (rad/s);
- Q: Air flow for aeration (m/s);
- λ1: Wavenumber (nm).
| Algorithm 1. Gaussian Elimination method |
| 1 Start 2 Declare the matrix. Where q is the number of unknown variables 3 Take the coefficients of the linear equation as: Do for k = 1 to n Do for j = 1 to n + 1 Read a[k][j] End for j End for k 4 Do for k = 1 to q − 1 Do for i = k + 1 to n Do for j = k + 1 to q + 1 a[i][j] = a[i][j] − a[i][k]/a[k][k] * a[k][j] End for j End for i End for k 5 Compute x[q] = a[q][q + 1]/a[q][q] 6 Do for k = q − 1 to 1 sum = 0 Do for j = k + 1 to q sum = sum + a[k][j] * x[j] End for j x[k] = 1/a[k][k] * (a[k][q + 1] − sum) End for k 7 Display the result x[k] 8 Stop |
3. Results and Discussion
3.1. Bioprocess
3.1.1. Estimated Effects
3.1.2. t-test and Analysis
3.1.3. Optimal Values of the Factors
3.2. Dimensional Analysis Using the Buckingham Pi Theorem
3.3. Experimental Results Obtained from the Correlation of the Mathematical Model
3.4. Results of the Mathematical Model
3.5. Bioprocess Scale-Up
- D: Bioreator diameter (m);
- Q: Bioreactor Airflow (m3/s).
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Markets and Markets. Bioprocess Validation Market by Test Type (Extractables and Leachables, Integrity Testing, Microbiology Testing), Process Component (Filter Element, Bioreactors), End-User (CDMO, Biotechnology & Pharmaceutical Companies)—Global Forecast to 2024; Markets and Markets: San Francisco, CA, USA, 2019. [Google Scholar]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Haringa, C.; Noorman, H.; Chu, J.; Zhuang, Y. Developing a Computational Framework To Advance Bioprocess Scale-Up. Trends Biotechnol. 2020, 38, 846–856. [Google Scholar] [CrossRef]
- Grand View Research. Industrial Bioprocessing Market Size, Share & Trends Analysis Report by Application, Regional Outlook, Competitive Strategies, and Segment Forecasts, 2019 to 2025; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Credence Research. Bioleaching Market by Type (Copper, Biomining, Iron) by Application (Mining, Others): Growth, Future Prospects, and Competitive Analysis, 2015–2027; Credence Research: San José, CA, USA, 2021. [Google Scholar]
- Kang, J.-K.; Cho, K.-H.; Kim, S.-B.; Choi, N.-C. Artificial Neural Network Modeling for Prediction of Dynamic Changes in Solution from Bioleaching by Indigenous Acidophilic Bacteria. Appl. Sci. 2020, 10, 7569. [Google Scholar] [CrossRef]
- Lalou, S.; Ferentidou, M.; Mantzouridou, F.T.; Tsimidou, M.Z. Balsamic type varietal vinegar from cv. Xinomavro (Northen Greece). Optimization and scale-up of the alcoholic fermentation step using indigenous multistarters. Food Chem. 2018, 244, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Enhancing the bioproduction of value-added aroma compounds via solid-state fermentation of sugarcane bagasse and sugar beet molasses: Operational strategies and scaling-up of the process. Bioresour. Technol. 2018, 263, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Hardy, N.; Augier, F.; Nienow, A.W.; Béal, C.; Ben Chaabane, F. Scale-up agitation criteria for Trichoderma reesei fermentation. Chem. Eng. Sci. 2017, 172, 158–168. [Google Scholar] [CrossRef]
- Sarkizi Shams Hajian, C.; Haringa, C.; Noorman, H.; Takors, R. Predicting By-Product Gradients of Baker’s Yeast Production at Industrial Scale: A Practical Simulation Approach. Processes 2020, 8, 1554. [Google Scholar] [CrossRef]
- Perez, C.L.; Casciatori, F.P.; Thoméo, J.C. Strategies for scaling-up packed-bed bioreactors for solid-state fermentation: The case of cellulolytic enzymes production by a thermophilic fungus. Chem. Eng. J. 2019, 361, 1142–1151. [Google Scholar] [CrossRef]
- de Medeiros, E.M.; Noorman, H.; Maciel Filho, R.; Posada, J.A. Production of ethanol fuel via syngas fermentation: Optimization of economic performance and energy efficiency. Chem. Eng. Sci. X 2020, 5, 100056. [Google Scholar] [CrossRef]
- Moreno-Zambrano, M.; Grimbs, S.; Ullrich, M.S.; Hütt, M.-T. A mathematical model of cocoa bean fermentation. R. Soc. Open Sci. 2018, 5, 180964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordas, M.; Konopacki, M.; Grygorcewicz, B.; Augustyniak, A.; Musik, D.; Wójcik, K.; Jędrzejczak-Silicka, M.; Rakoczy, R. Hydrodynamics and Mass Transfer Analysis in BioFlow® Bioreactor Systems. Processes 2020, 8, 1311. [Google Scholar] [CrossRef]
- Rodman, A.D.; Gerogiorgis, D.I. Computer Aided Chemical Engineering; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 38, pp. 1033–1038. [Google Scholar]
- Tang, R.C.O.; Jang, J.H.; Lan, T.H.; Wu, J.C.; Yan, W.M.; Sangeetha, T.; Wang, C.T.; Ong, H.C.; Ong, Z.C. Review on design factors of microbial fuel cells using Buckingham’s Pi Theorem. Renew. Sustain. Energy Rev. 2020, 130, 109878. [Google Scholar] [CrossRef]
- Russo, L.; Sorrentino, M.; Polverino, P.; Pianese, C. Application of Buckingham Π theorem for scaling-up oriented fast modelling of Proton Exchange Membrane Fuel Cell impedance. J. Power Sources 2017, 353, 277–286. [Google Scholar] [CrossRef]
- Polverino, P.; Bove, G.; Sorrentino, M.; Pianese, C.; Beretta, D. Advancements on scaling-up simulation of Proton Exchange Membrane Fuel Cells impedance through Buckingham Pi theorem. Appl. Energy 2019, 249, 245–252. [Google Scholar] [CrossRef]
- Ramirez-Carmona, M.; Cardona, M.; Torres-Taborda, M. Introducción a la ingeniería Bioquímica; Universidad Pontificia Bolivariana: Medellin, Colombia, 2009. [Google Scholar]
- Montgomery, D.; Runger, G. Applied Statistics and Probability for Engineers; John Wyley & Sons Inc.: Danvers, MA, USA, 2010. [Google Scholar]
- Carmona, M.E.R.; da Silva, M.A.P.; Ferreira Leite, S.G. Biosorption of chromium using factorial experimental design. Process Biochem. 2005, 40, 779–788. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef]
- Hofmann, T.; Shaw, R. Proteolytic enzymes of Penicillium janthinellum. I. Purification and properties of a trypsinogen-activating enzyme (peptidase A). BBA-Enzymol. Subj. 1964, 92, 543–557. [Google Scholar]
- Valix, M. Method for Removing Boron in Water Treatment. US20110259829A1, 2009. Available online: https://patentimages.storage.googleapis.com/a5/ab/60/e8c229258db8b3/US20110259829A1.pdf (accessed on 20 November 2021).
- Shadafza, D.; Ogawa, T.; Fazeli, A. Comparison of citric acid production from beet molasses and date syrup withAspergillus niger. Hakko Kogaku Zasshi 1976, 54, 65–75. [Google Scholar]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem. 2003, 38, 1731–1738. [Google Scholar] [CrossRef]
- Rokosu, A.A.; Anenih, C.A. Effects of various conditions on the production of citric acid during fermentation of molasses by Aspergillus niger. Enzyme Microb. Technol. 1980, 2, 61–62. [Google Scholar] [CrossRef]
- Roukas, T.; Harvey, L. The effect of pH on production of citric and gluconic acid from beet molasses using continuous culture. Biotechnol. Lett. 1988, 10, 289–294. [Google Scholar] [CrossRef]
- Le, H.; Kabbur, S.; Pollastrini, L.; Sun, Z.; Mills, K.; Johnson, K.; Karypis, G.; Hu, W.S. Multivariate analysis of cell culture bioprocess data—Lactate consumption as process indicator. J. Biotechnol. 2012, 162, 210–223. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Scale-up of photo-bioreactors for microalgae cultivation by π-theorem. Biochem. Eng. J. 2020, 153, 107398. [Google Scholar] [CrossRef]
- Harun, N.H.; Abdul-Aziz, A.; Rahman, R.A.; Yaakub, H.; Aziz, R.; Ware, I. Dimensional-analysis and similitude for scale-up of solid-liquid extraction of Eurycoma longifolia roots. Chem. Eng. Sci. 2020, 217, 115490. [Google Scholar] [CrossRef]
- Acevedo, F. The use of reactors in biomining processes. Electron. J. Biotechnol. 1997, 3, 10–11. [Google Scholar] [CrossRef]
- Klöckner, W.; Tissot, S.; Wurm, F.; Büchs, J. Power input correlation to characterize the hydrodynamics of cylindrical orbitally shaken bioreactors. Biochem. Eng. J. 2012, 65, 63–69. [Google Scholar] [CrossRef]
- Pandey, P.; Putatunda, S.; Dewanga, L.; Pawar, V.; Belorkar, S. Studies on citric acid production by Aspergillus niger in batch fermentation. Recent Res. Sci. Technol. 2013, 5, 66–67. [Google Scholar]
- Aboyeji, O.O.; Oloke, J.K.; Arinkoola, A.O.; Oke, M.A.; Ishola, M.M. Optimization of media components and fermentation conditions for citric acid production from sweet potato peel starch hydrolysate by Aspergillus niger. Sci. Afr. 2020, 10, e00554. [Google Scholar] [CrossRef]
- Pritchard, P. Fox and McDonald’s Introduction to Fluid Mechanics; John Wiley & Sons, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Platon, R.R. Oxygen Mass Transfer Coefficient (K L a) as Scale-Up Criterion in Brine Shrimp Culture. IFAC Proc. Vol. 1987, 20, 109–117. [Google Scholar] [CrossRef]







| Experiment Number | N Source | C Source | K Source | Trace Elements | Agitation |
|---|---|---|---|---|---|
| 1 | Urea (0.75 g/L) | Sucrose (120 g/L) | K2HPO4 (0.128 g/L) | No | Mechanical |
| 2 | (NH4)2SO4 (0.428 g/L) | Sucrose (40 g/L) | KH2PO4 (0.8 g/L) | No | Mechanical |
| 3 | (NH4)2SO4 (0.428 g/L) | Sucrose (120 g/L) | KH2PO4 (0.8 g/L) | Yes | Mechanical |
| 4 | Urea (0.75 g/L) | Sucrose (40 g/L) | KH2PO4 (0.8 g/L) | Yes | Aeration mixing |
| 5 | (NH4)2SO4 (0.428 g/L) | Sucrose (120 g/L) | K2HPO4 (0.128 g/L) | Yes | Aeration mixing |
| 6 | Urea (0.75 g/L) | Sucrose (40 g/L) | K2HPO4 (0.128 g/L) | Yes | Mechanical |
| 7 | Urea (0.75 g/L) | Sucrose (120 g/L) | KH2PO4 (0.8 g/L) | No | Aeration mixing |
| 8 | (NH4)2SO4 (0.428 g/L) | Sucrose (40 g/L) | K2HPO4 (0.128 g/L) | No | Aeration mixing |
| Experiment | Factors | Response Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Source (FN) | C Source (FC) | K Source (FK) | Trace Elements | Agitation | pH | A1 (g/L) | A2 (g/L) | A3 (g/L) | A4 (g/L) | λ1 1060 cm−1 | λ2 1456 cm−1 | |
| 1 | Urea (0.75 g/L) | Sucrose (120 g/L) | K2HPO4 (0.128 g/L) | No | Mechanical | 3.192 | 0.222 | 0.252 | 6.503 | 0.034 | 0.095 | 0.0720 |
| 2 | (NH4)2SO4 (0.428 g/L) | Sucrose (40 g/L) | KH2PO4 (0.8 g/L) | No | Mechanical | 2.820 | 0.009 | 0.291 | 0.964 | 0.011 | 0.066 | 0.0630 |
| 3 | (NH4)2SO4 (0.428 g/L) | Sucrose (120 g/L) | KH2PO4 (0.8 g/L) | Yes | Mechanical | 2.942 | 0.002 | 0.101 | 5.627 | 0.024 | 0.098 | 0.0686 |
| 4 | Urea (0.75 g/L) | Sucrose (40 g/L) | KH2PO4 (0.8 g/L) | Yes | Aeration mixing | 3.119 | 0.002 | 0.019 | 3.470 | 0.102 | 0.053 | 0.0599 |
| 5 | (NH4)2SO4 (0.428 g/L) | Sucrose (120 g/L) | K2HPO4 (0.128 g/L) | Yes | Aeration mixing | 2.552 | 0.006 | 0.107 | 6.091 | 0.131 | 0.096 | 0.0683 |
| 6 | Urea (0.75 g/L) | Sucrose (40 g/L) | K2HPO4 (0.128 g/L) | Yes | Mechanical | 3.152 | 0.007 | 0.784 | 0.972 | 0.030 | 0.063 | 0.0614 |
| 7 | Urea (0.75 g/L) | Sucrose (120 g/L) | KH2PO4 (0.8 g/L) | No | Aeration mixing | 2.771 | 0.015 | 0.428 | 3.446 | 0.036 | 0.066 | 0.0672 |
| 8 | (NH4)2SO4 (0.428 g/L) | Sucrose (40 g/L) | K2HPO4 (0.128 g/L) | No | Aeration mixing | 2.472 | 0.007 | 0.140 | 1.177 | 0.117 | 0.068 | 0.0638 |
| Factor | Factor Bioleaching of Gold Using the Infrared Spectrum at 1060 cm−1 | Factor Bioleaching of Gold Using the Infrared Spectrum at 1456 cm−1 |
|---|---|---|
| Estimated ± standard error | Estimated ± standard error | |
| Average | 0.0749 ± 0.00061 | 0.0647 ± 0.000441 |
| A: FN + BD + CE | 0.0133 ± 0.00122 | 0.0020 ± 0.000883 |
| B: FC + AD | 0.0255 ± 0.00122 | 0.0064 ± 0.000883 |
| C: FK + AE | −0.0093 ± 0.00122 | −0.0016 ± 0.000883 |
| D: Trace + AB | 0.0043 ± 0.00122 | −0.0009 ± 0.000883 |
| E: Mixing + AC | 0.0118 ± 0.00122 | 0.0018 ± 0.000883 |
| BC + DE | −0.0046 ± 0.00122 | −0.0006 ± 0.000883 |
| BE + CD | 0.0047 ± 0.00122 | 0.0003 ± 0.000883 |
| Factor | Factor Bioleaching of Gold Using the Infrared Spectrum at 1060 cm−1 (R2 = 0.9895) | Factor Bioleaching of Gold Using the Infrared Spectrum at 1456 cm−1 (R2 = 0.8931) | ||
|---|---|---|---|---|
| Coded | Level | Coded | Level | |
| FN (g/L) | 0.96 | Urea | 1.0 | Urea |
| FC (g/L) | 0.97 | 120 | 0.97 | 120 |
| FK (g/L) | −1.0 | K2HPO4 | −1.0 | K2HPO4 |
| Traces | 1.0 | Yes | −0.99 | No |
| Agitation | 0.92 | Mechanical | 0.98 | Mechanical |
| Experiment Number | N Source | C Source | K Source |
|---|---|---|---|
| Carbon Source Concentration | C | kg m−3 | ML−3 |
| Nitrogen source concentration | N | kg m−3 | ML−3 |
| Potassium source concentration | K | kg m−3 | ML−3 |
| Trace concentration | T | kg m−3 | ML−3 |
| Agitation | A | rad s−1 | T−1 |
| Air flow for aeration | Q | m3 s−1 | L3T−1 |
| Wavelength | λ1 | m | L |
| Experiment | C/N Ratio | C/K Ratio | T/C Ratio | Absorbance (1060 cm−1) |
|---|---|---|---|---|
| 1 | 160.00 | 937.50 | 0.00 | 0.0952 |
| 2 | 93.46 | 50.00 | 0.00 | 0.0656 |
| 3 | 280.37 | 150.00 | 208.33 | 0.0981 |
| 4 | 53.33 | 50.00 | 625.00 | 0.0532 |
| 5 | 280.37 | 937.50 | 208.33 | 0.0961 |
| 6 | 53.33 | 312.50 | 625.00 | 0.0630 |
| 7 | 160.00 | 150.00 | 0.00 | 0.0662 |
| 8 | 93.46 | 312.50 | 0.00 | 0.0681 |
| Model Coefficient | Mean Value | Min Value | Max Value |
|---|---|---|---|
| 6.307 × 10−1 | 5.32997 × 10−1 | 7.28467 × 10−1 | |
| −8.941 × 10−4 | −1.12021 × 10−3 | −6.68058 × 10−4 | |
| −5.181 | −6.06245 | −4.29899 | |
| 4.233 × 10−7 | 1.64967 × 10−7 | 6.81627 × 10−7 | |
| 11.07 | 9.14895 | 13.0008 | |
| 4.950 × 10−3 | 3.98539 × 10−3 | 5.91491 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Mathematical Model for Scaling up Bioprocesses Using Experiment Design Combined with Buckingham Pi Theorem. Appl. Sci. 2021, 11, 11338. https://doi.org/10.3390/app112311338
Rendón-Castrillón L, Ramírez-Carmona M, Ocampo-López C, Gómez-Arroyave L. Mathematical Model for Scaling up Bioprocesses Using Experiment Design Combined with Buckingham Pi Theorem. Applied Sciences. 2021; 11(23):11338. https://doi.org/10.3390/app112311338
Chicago/Turabian StyleRendón-Castrillón, Leidy, Margarita Ramírez-Carmona, Carlos Ocampo-López, and Luis Gómez-Arroyave. 2021. "Mathematical Model for Scaling up Bioprocesses Using Experiment Design Combined with Buckingham Pi Theorem" Applied Sciences 11, no. 23: 11338. https://doi.org/10.3390/app112311338