Parallel Evolution of Allometric Trajectories of Trophic Morphology between Sympatric Morphs of Mesoamerican Astyanax (Characidae)
Abstract
:1. Introduction
2. Methods
2.1. Sampling Collection
2.2. Premaxilla Shape Variation
2.3. Ontogenetic Allometric Trajectories
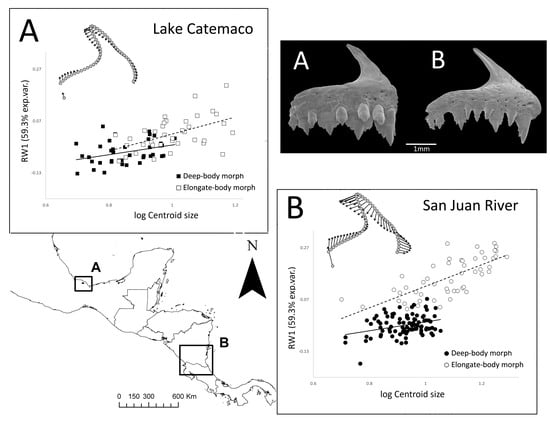
3. Results
3.1. Premaxilla Shape Variation
3.2. Ontogenetic Allometric Trajectories
4. Discussion
4.1. Premaxilla Shape Divergence between Morphs and Systems
4.2. Patterns of Allometric Ontogenetic Trajectories between Morphs and Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, S.J. Allometry and size in ontogeny and phylogeny. Biol. Rev. 1966, 41, 587–638. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Multivariate Allometry. In Advances in Morphometrics; NATO ASI Series; Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; Springer: Boston, MA, USA, 1996; pp. 23–49. [Google Scholar]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Sabath, N.; Pyron, R.A.; Mayrose, I.; Meiri, S. Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Glob. Ecol. Biogeogr. 2016, 25, 187–197. [Google Scholar] [CrossRef]
- Friedman, S.T.; Martinez, C.M.; Price, S.A.; Wainwright, P.C. The influence of size on body shape diversification across Indo-Pacific shore fishes. Evolution 2019, 73, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Pélabon, C.; Firmat, C.; Bolstad, G.H.; Voje, K.L.; Houle, D.; Cassara, J.; Le Rouzic, A.; Hansen, T.F. Evolution of morphological allometry. Ann. N. Y. Acad. Sci. 2014, 1320, 58–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svanbäck, R.; Eklöv, P. Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 2002, 131, 61–70. [Google Scholar] [CrossRef]
- Hollander, J.; Adams, D.C.; Johannesson, K. Evolution of adaptation through allometric shifts in a marine snail. Evolution 2006, 60, 2490–2497. [Google Scholar] [CrossRef]
- Piras, P.; Salvi, D.; Ferrara, G.; Maiorino, L.; Delfino, M.; Pedde, L.; Kotsakis, T. The role of post-natal ontogeny in the evolution of phenotypic diversity in Podarcis lizards. J. Evol. Biol. 2011, 24, 2705–2720. [Google Scholar] [CrossRef]
- Esquerré, D.; Sherratt, E.; Keogh, J.S. Evolution of extreme ontogenetic allometric diversity and heterochrony in pythons, a clade of giant and dwarf snakes. Evolution 2017, 71, 2829–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsen, M.K.; Siwertsson, A.; Adams, C.E.; Amundsen, P.A.; Præbel, K.; Knudsen, R. Allometric trajectories of body and head morphology in three sympatric Arctic charr (Salvelinus alpinus (L.)) morphs. Ecol. Evol. 2017, 7, 7277–7289. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.A.; Sherratt, E.; Hutchinson, M.N.; Jones, M.E. Changes in ontogenetic patterns facilitate diversification in skull shape of Australian agamid lizards. BMC Evol. Biol. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingenberg, C.P. There’s something afoot in the evolution of ontogenies. BMC Evol. Biol. 2010, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.B.; Borowsky, R.; Tabin, C.J. A novel role for Mc1r in the parallel evolution of depigmentation in independent populations of the cavefish Astyanax mexicanus. PLoS Genet. 2009, 5, e1000326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, W.R. Regressive evolution in Astyanax cavefish. Annu. Rev. Genet. 2009, 43, 25–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elipot, Y.; Hinaux, H.; Callebert, J.; Launay, J.M.; Blin, M.; Rétaux, S. A mutation in the enzyme monoamine oxidase explains part of the Astyanax cavefish behavioural syndrome. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGaugh, S.E.; Gross, J.B.; Aken, B.; Blin, M.; Borowsky, R.; Chalopin, D.; Hinaux, H.; Jeffery, W.R.; Keene, A.C.; Ma, L.; et al. The cavefish genome reveals candidate genes for eye loss. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornelas-García, C.P.; Domínguez-Domínguez, O.; Doadrio, I. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol. Biol. 2008, 8, 340. [Google Scholar]
- Ornelas-García, C.P.; Bastir, M.; Doadrio, I. Morphometric variation between two morphotypes within the Astyanax Baird and Girard, 1854 (Actinopterygii: Characidae) genus, From a Mexican tropical lake. J. Morphol. 2014, 275, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Ornelas-García, C.P.; Córdova-Tapia, F.; Zambrano, L.; Bermúdez-González, M.P.; Mercado-Silva, N.; Mendoza-Garfias, B.; Bautista, A. Trophic specialization and morphological divergence between two sympatric species in Lake Catemaco, Mexico. Ecol. Evol. 2018, 8, 4867–4875. [Google Scholar] [CrossRef]
- Garita-Alvarado, C.A.; Barluenga, M.; Ornelas-García, C.P. Parallel evolution of morphs of Astyanax species (Teleostei: Characidae) in México and Central America. Biol. J. Linn. Soc. 2018, 124, 706–717. [Google Scholar] [CrossRef]
- Powers, A.K.; Garita-Alvarado, C.A.; Rodiles-Hernández, R.; Berning, D.J.; Gross, J.B.; Ornelas-García, C.P. A geographical cline in craniofacial morphology across populations of Mesoamerican lake-dwelling fishes. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 171–180. [Google Scholar] [CrossRef]
- Ornelas-García, C.P.; Gonzalez, E.; Tautz, D.; Doadrio, I. Lack of genetic differentiation between two sympatric species of Astyanax (Characidae: Teleostei) in Lake Catemaco, Mexico. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Garita-Alvarado, C.A.; Garduño-Sánchez, M.; Barluenga, M.; Ornelas-García, C.P. Genetic and ecomorphological divergence between sympatric Astyanax morphs from Central America. bioRxiv 2021. [Google Scholar] [CrossRef]
- Contreras-Balderas, S.; Rivera-Teillery, R. Bramocharax (Catemaco) caballeroi Subgen. et. sp. nv., del lago de Catemaco, Veracruz, México. Publ. Biológicas Inst. Investig. Científicas UANL 1985, 2, 7–29. [Google Scholar]
- Bussing, W.A. Freshwater Fishes of Costa Rica, 1st ed.; Universidad de Costa Rica: San José, CA, USA, 1998. [Google Scholar]
- Sidlauskas, B. Testing for unequal rates of morphological diversification in the absence of a detailed phylogeny: A case study from Characiform fishes. Evolution 2007, 61, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biol. Rev. 1998, 73, 79–123. [Google Scholar] [CrossRef] [PubMed]
- Foote, M. Contributions of individual taxa to overall morphological disparity. Paleobiology 1993, 19, 403–419. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix Ital. J. Mammal. 2015, 26, 9–12. [Google Scholar]
- De Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Nosil, P.; Harmon, L.J.; Seehausen, O. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 2009, 24, 145–156. [Google Scholar] [CrossRef]
- Cooper, W.J.; Parsons, K.; McIntyre, A.; Kern, B.; McGee-Moore, A.; Albertson, R.C. Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PLoS ONE 2010, 5, e9551. [Google Scholar] [CrossRef] [PubMed]
- Sheets, H.D.; Zelditch, M.L. Studying ontogenetic trajectories using resampling methods and landmark data. Hystrix Ital. J. Mammal. 2013, 24, 67–73. [Google Scholar]
- Cardini, A.; O’Higgins, P. Post-natal ontogeny of the mandible and ventral cranium in Marmota species (Rodentia, Sciuridae): Allometry and phylogeny. Zoomorphology 2005, 124, 189–203. [Google Scholar] [CrossRef]
- Urošević, A.; Ljubisavljević, K.; Ivanović, A. Patterns of cranial ontogeny in lacertid lizards: Morphological and allometric disparity. J. Evol. Biol. 2013, 26, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Duport-Bru, A.S.; Ponssa, M.L.; Vera Candioti, F. Postmetamorphic ontogenetic allometry and the evolution of skull shape in Nest-building frogs Leptodactylus (Anura: Leptodactylidae). Evol. Dev. 2019, 21, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.S.; Vliet, K.A.; Abzhanov, A.; Pierce, S.E. Heterochronic shifts and conserved embryonic shape underlie crocodylian craniofacial disparity and convergence. Proc. R. Soc. B 2019, 286, 20182389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, M.V. Morphological diversification of fish as a consequence of the divergence of ontogenetic trajectories. Russ. J. Dev. Biol. 2001, 32, 397–402. [Google Scholar] [CrossRef]
- Corse, E.; Neve, G.; Sinama, M.; Pech, N.; Costedoat, C.; Chappaz, R.; Andre, G. Plasticity of ontogenetic trajectories in cyprinids: A source of evolutionary novelties. Biol. J. Linn. Soc. 2012, 106, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.C.; Menezes, N.A. Phylogenetic relationships of the species and biogeography of the characid genus Oligosarcus Günther, 1864 (Ostariophysi, Characiformes, Characidae). Zootaxa 2015, 3949, 41–81. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.; Yamamoto, Y.; Stock, D.W. Ontogenetic transition from unicuspid to multicuspid oral dentition in a teleost fish: Astyanax mexicanus, the Mexican tetra (Ostariophysi: Characidae). Zool. J. Linn. Soc. 2005, 145, 523–538. [Google Scholar] [CrossRef]
- Atukorala, A.D.S.; Franz-Odendaal, T.A. Spatial and temporal events in tooth development of Astyanax Mex. Mech. Dev. 2014, 134, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Tabin, C.J. Introduction: The Emergence of the Mexican Cavefish as an Important Model System for Understanding Phenotypic Evolution. In Biology and Evolution of the Mexican Cavefish; Keene, A., Yoshizawa, M., McGaugh, S., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–5. [Google Scholar]




| System | Lake/River | Sampling Site | Site Number | Deep-Body | Elongate-Body |
|---|---|---|---|---|---|
| Lake Catemaco | Lake Catemaco | Market | 1 | - | 7 |
| Coyame | 2 | 22 | 14 | ||
| Tebanca | 3 | 17 | 16 | ||
| San Juan River | Lake Managua | Puerto Momotombo | 4 | 1 | 1 |
| South of Momotombo volcano | 5 | 7 | 7 | ||
| Isla Escondida | 6 | 6 | 2 | ||
| Lake Nicaragua | Isletas de Granada | 7 | 23 | 9 | |
| Ometepe | 8 | 19 | 6 | ||
| Solentiname | 9 | 1 | 1 | ||
| Sarapiquí River | Tambor | 10 | 18 | 24 | |
| Tirimbina | 11 | 16 | 1 |
| System/Morph | RW1 | RW2 | ||||
|---|---|---|---|---|---|---|
| Slope | R2 | p | Slope | R2 | p | |
| Lake Catemaco | ||||||
| Deep-body | 0.19 | 0.13 | 0.02 | 0.03 | 0.004 | 0.69 |
| Elongate-body | 0.33 | 0.26 | 0.001 | 0.00001 | <0.001 | 0.99 |
| San Juan River | ||||||
| Deep-body | 0.17 | 0.09 | 0.002 | 0.21 | 0.09 | 0.003 |
| Elongate-body | 0.42 | 0.52 | <0.001 | 0.004 | <0.001 | 0.94 |
| Factor | RW1 | RW2 | ||||
|---|---|---|---|---|---|---|
| Sum of Squares | F | p | Sum of Squares | F | p | |
| Lake Catemaco | ||||||
| Log Centroid size | 0.13 | 53.41 | <0.001 | 0.001 | 0.58 | 0.44 |
| Morph | 0.011 | 4.68 | 0.03 | 0.004 | 2.01 | 0.16 |
| Morph*log Centroid size | 0.003 | 1.25 | 0.26 | 0.0002 | 0.11 | 0.73 |
| San Juan River | ||||||
| Log Centroid size | 0.89 | 459.9 | <0.001 | 0.046 | 18.86 | <0.001 |
| Morph | 0.367 | 188.95 | <0.001 | 0.016 | 6.66 | 0.01 |
| Morph*log Centroid size | 0.019 | 10.15 | 0.001 | 0.013 | 5.44 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garita-Alvarado, C.A.; Ornelas-García, C.P. Parallel Evolution of Allometric Trajectories of Trophic Morphology between Sympatric Morphs of Mesoamerican Astyanax (Characidae). Appl. Sci. 2021, 11, 8020. https://doi.org/10.3390/app11178020
Garita-Alvarado CA, Ornelas-García CP. Parallel Evolution of Allometric Trajectories of Trophic Morphology between Sympatric Morphs of Mesoamerican Astyanax (Characidae). Applied Sciences. 2021; 11(17):8020. https://doi.org/10.3390/app11178020
Chicago/Turabian StyleGarita-Alvarado, Carlos A., and Claudia Patricia Ornelas-García. 2021. "Parallel Evolution of Allometric Trajectories of Trophic Morphology between Sympatric Morphs of Mesoamerican Astyanax (Characidae)" Applied Sciences 11, no. 17: 8020. https://doi.org/10.3390/app11178020