1. Introduction
The increasing demand for energy and the limited resources of fossil fuels have forced humans to look for renewable energy alternatives to fossil fuels [
1,
2]. Fischer-Tropsch synthesis (FTS) uses transition metals, such as cobalt (Co), iron (Fe), and nickel (Ni), as catalysts [
3,
4,
5,
6] to make non-petroleum raw materials (mostly coal, biomass, and natural gas). Syngas (a mixture of CO and H
2) is converted into pollution-free fuel and valuable chemicals. It offers a way to convert coal or natural gas into gasoline, diesel fuel, and other useful hydrocarbons such as waxes [
7,
8,
9,
10]. However, FTS technology has led to the formation of a large amount of Fischer-Tropsch wax residue (FTWR) and has caused serious industrial pollution and public land occupation problems.
FTWR is a typical wax-based solid waste mainly from the wax filter unit of FTS. Different wax filter units have various FTWR components, which are mostly composed of 40–80 wt% Fischer-Tropsch wax (FTW), 30–85 wt% Fischer-Tropsch catalyst (FTC), and 0–80 wt% minerals and other impurities [
7]. In many countries, FTWR is classified as hazardous waste. If improperly handled, it will produce toxic and hazardous substances [
11,
12], causing damage to the ecological environment and posing a threat to human health. Reports have reported that approximately 20,000 tons of slag wax are produced in China each year, 60% of the slag wax is incinerated, and the rest is used for landfill [
13]. However, incineration and landfill treatment waste many FTW resources in FTWR, and the organic matter produced by incineration pollutes the atmosphere [
14,
15]. Landfill disposal will take up a lot of land resources, cause leachate to seep out, thereby polluting soil and groundwater, and will cause high economic costs and other problems [
16]. Recycling the valuable resources in FTWR, avoiding resource waste, and polluting the environment is the correct way to dispose of FTWR.
Solvent desorption is a commonly used method to recover lipids from oily waste [
17,
18,
19]. It has been widely used to recover aliphatic hydrocarbons from oily sludge and other oily wastes [
20,
21]. Mohit et al. studied the combination of MEK (polar solvent) and xylene (non-polar solvent) as a mixed solvent for the recovery of hydrocarbons from oil sludge, showing that xylene has a good recovery of hydrocarbons in petroleum efficiency [
22]. Nour et al. used isopropanol solvent to extract waste engine oil to obtain regenerated oil [
23]. Many studies have investigated solvent desorption to recover oily wastes containing sludge, but few have reported the recycling of FTW resources in FTWR. In fact, the current resource recovery method for wax-based solid waste is mainly pyrolysis, which converts wax into pyrolysis gas and then condenses and recovers it. The presence of the Fischer-Tropsch catalyst in FTWR affect the quality of the finished wax recovered by pyrolysis. As a result, the carbon number of the finished wax and the recovery rate were poor. This will inevitably waste a part of wax resources. The energy requirements of the pyrolysis method were also far greater than that of the solvent method.
In this study, wax-based solid waste was compared with oily solid waste, petroleum ether solvent was used to separate recycle Fischer-Tropsch wax (RFTW) from FTWR efficiently to study the influence of several factors, such as solvent/FTWR mass ratio, reaction residence time, and reaction temperature on the product yield. The product recovery rate is obtained by measuring the mass change of FTWR before and after the reaction. Central composite design-response surface methodology (CCD-RSM) was used to screen the best process conditions for FTW desorption. The desorption process of the wax in the three-way environment of Fischer-Tropsch wax, solvent and catalyst was verified and analyzed through isotherm analysis and kinetic fitting, and the desorption mechanism was proposed. Through BET, BJH, SEM morphology analysis and other characterization were used to verify the mechanism. Furthermore, the recovered products were characterized and analyzed by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and Gas chromatography-mass spectrometer (GC-MS). This study provides a new direction for the harmless disposal and resource utilization of FTWR. All in all, this work was devoted to finding the best extraction process and elucidating the reaction mechanism through systematic experimental and theoretical methods.
2. Material and Methods
FTWR was obtained from a Chinese coal company (Ningxia, China). The desorption solvent petroleum ether was provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The elemental analysis of FTWR was performed by X-ray fluorescence spectrometry (XRF, Eagle III, EDAX Inc., San Diego, CA, USA). DESIGN EXPERT 12 (Minneapolis, USA) was used to design and extract the experiment and establish a mathematical model of the relationship between each factor and the response value to determine the best research conditions.
2.1. Properties of FTWR
The element composition of FTWR were analyzed, and the results are shown in
Table 1.
2.2. Design of Experimental Condition Using RSM
RSM is considered a mathematical model that can accurately reveal study the relationship between various factors and response values [
24,
25,
26]. It can quickly and effectively determine the best conditions for a multi-factor system [
27,
28,
29]. A five-level, three-factor CCD technique was employed to optimize the independent process variables. Temperature (A), retention time (B), and liquid–solid ratio (C) in the range of 25–80 °C, 0.5–4 h, and 10–30, respectively, were considered as independent process parameters, and extracted wax yield was regarded as the desired response that needs to be maximized.
Table 2 shows the coding level of the experimental variables used in the CCD method.
First, establishing a mathematical model of independent variables and response values and using Design Expert 12 software are necessary to fit the RSM model according to Equation (1) using the least square method:
where
Y signifies the predicted value of the response and
,
,
, and
are the constant term and coefficient for linear, quadratic, and interaction terms, respectively, in the developed model equation.
k signifies the number of independent process variables chosen for process optimization (
k = 4).
Second, ANOVA was used to analyze the optimization results, and the product desorption rate obtained in the actual experiment was used as the real value. The predicted value was obtained from the software (Design Expert 12, Minneapolis, MIN, USA). Analysis of the data through ANOVA shows the analysis of variance, the significance of variance test, and the first-order coefficient significance test regression equation. The reliability of the model is tested [
28,
29,
30,
31].
2.3. Solvent Extraction Experiments
To reduce the experimental error and improve the accuracy of the experiment, pre-processing the FTWR is necessary. The pretreatment steps are as follows: (1) FTWR was putted in a drying oven and dry it at 80 °C for 8 h; (2) the dried FTWR was grinded and crushed into small particles and pass it through a 120-mesh screen to obtain a FTWR with a particle size of less than 134 μm. FTWR after grinding and sieving was used in the following experiments. Typically, the desorption experiment was carried out according to the RSM response surface optimization design plan. The required mass of FTWR is accurately weighed, FTWR wrapped with filter paper, and placed in a 100 mL flask. The necessary volume of petroleum ether is then added to the flask. The flask was later placed on a constant temperature shaker (SHA-B, GUOHUA, Changzhou, China) to complete the design conditions’ entire reaction process. The solvent in the washed extract was evaporated on a rotary evaporator. Finally, the residue was weighed and named as Recycle Fischer-Tropsch wax (RFTW). The residues after FTWR leaching were dried and weighed to calculate the product yield. Equation (2) was used to calculate the yield of the extracted product in FTWR. Each experiment was repeated three times, and the results were averaged.
where
represents the recovery rate of RFTW,
M and
m, respectively, represent the mass of FTWR before and after treatments (g), α represents the mass percentage of carbon-containing compounds in FTWR obtained by XRF analysis, and α = 0.4082 in this experiment.
2.4. Material Characterization
Specific surface area and detailed porosity parameters (pore size distribution and pore volume) were determined using the Brunauer–Emmett–Teller (BET) and the Barrett–Joyner–Halenda plot methods. The nitrogen adsorption and desorption curves of FTWR were also obtained. Morphology was characterized by using a scanning electron microscope (SEM; S4800, Hitachi Corporation, Tokyo, Japan). An X-ray diffractometer (Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA) was used to collect XRD data of RFTW with monochromatic Cu/Kα radiation (λ = 1.54056 Å) at 36 kV and 20 mA in a 2 θ scanning range of 20–70°. The types of functional groups in RFTW were analyzed by NEXUS870 Fourier Transform Infrared Spectrometer (FT-IR) (Nicolet 8700, Thermo Fisher Scientific, Waltham, USA). The scanning was done from 400 cm−1 to 4000 cm−1 at a scanning rate of 40 with the step size of 4 cm−1 by adopting attenuated total reflectance. Gas chromatography-mass spectrometry (GC-MS) (Shimadzu Corporation, Tokyo, Japan) was used to analysis the components of samples. The capillary column used VF-5MS (30 m × 0.25 mm; 0.25 μm), the carrier gas was helium, and the flow rate was 0.8 mL/min. The analysis was performed in duplicate by injecting 1 μL in splitless mode. The sampler was set to 280 °C, the transfer line was set to 280 °C, and the ion source was set to 300 °C. The column was initially set to 100 °C for 1 min, heated at 10 °C/min to 200 °C and 200 °C for 2 min, and finally reheated at 3.5 °C/min until 260 °C. The total analysis time was 40 min. The mass spectrum was collected every 0.5 s with a range of 32−380 m/z. In this way, qualitative and quantitative analysis of RFTW was carried out.
4. Conclusions
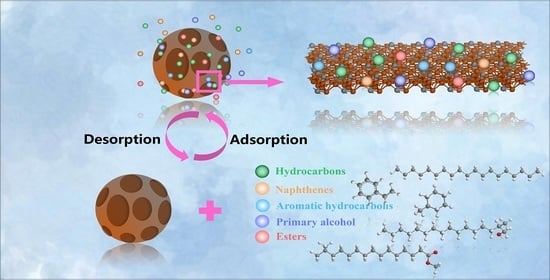
The wax from FTWR was obtained by solvent extraction with petroleum ether. Through the RSM mathematical model prediction, the optimal process conditions were obtained, and the actual experiment verified that the product yield under the optimal reaction conditions could reach 82.28%. As the temperature increased, it was beneficial to the thermal movement of FTW molecules, thereby destroying the limiting effect of FTC and increasing the recovery rate. The recovery rate decreased with the increase of the liquid–solid ratio, which was attributed to the particle concentration effect which limited the continuous increase of the recovery rate. In addition, the petroleum ether solvent has similar compatibility with the recovered wax, which was conducive to the desorption process.
The N2 adsorption-desorption isotherm could better define the desorption process, indicated the multi-molecular layer adsorption and desorption behavior of FTWR. The thermodynamic function results showed that there were not only van der Waals forces in the desorption process, but other strong interaction forces such as hydrogen bonds. In addition, the desorption process could be well described by quasi-second-order kinetics and internal diffusion models. This indicated that the desorption behavior on FTWR was mainly controlled by chemical desorption. Therefore, the desorption process of FTWR could be described as: The FTW on the FTC surface was first desorbed into the solvent due to the principle of compatibility; then, since the interaction between FTW molecules is greater than the force of the pores in the FTC on the FTW molecules. It also gradually desorbs into the solvent to obtain RFTW and WFTC. Hydrogen bonds and other strong interaction forces are the key to the desorption process.
This research solved the problem of high economic cost and waste of resources for FTWR as a hazardous waste disposal and provided an economical and feasible method for the resource utilization of FTWR. The high-quality wax recovered in the process can be further purified and used in other industrial fields to achieve the goal of waste recycling. In addition, this study provided theoretical guidance for a better understanding of the wax desorption process in FTWR.