Surface Modification of a Graphite Felt Cathode with Amide-Coupling Enhances the Electron Uptake of Rhodobacter sphaeroides
Abstract
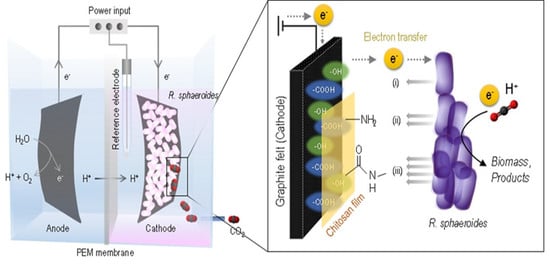
:1. Introduction
2. Materials and Methods
2.1. Strain and Medium Preparation
2.2. Microbial Electrosynthesis Reactor Configuration and Operation
2.3. Electrode Modification Procedure
2.4. Analytical Methods
2.5. Calculation of Faradaic Efficiency
CH1.99O0.50N0.19 + 0.095H2SO4 + 1.5H2O
3. Results and Discussion
3.1. Modification of the Graphite Felt Electrode for Amide-Coupling
3.2. Electrochemical Evaluation of Chitosan- and Chitosan/Carbodiimide Compound-Modification
3.3. MES Operation and Biofilm Morphology of Biocathodes
3.4. Enhanced CO2 Conversion by Modified Cathodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Oh, Y.-K.; Lee, S.; Fitriana, H.N.; Moon, M.; Kim, M.-S.; Lee, J.; Min, K.; Park, G.W.; Lee, J.-P.; et al. Recent developments and key barriers to microbial CO2 electrobiorefinery. Bioreousr. Technol. 2021, 320, 12450. [Google Scholar] [CrossRef]
- Chatzipanagiotou, K.-R.; Soekhoe, V.; Jourdin, L.; Buisman, C.J.N.; Bitter, J.H.; Strik, D.P.B.T.B. Catalytic cooperation between a copper oxide electrocatalyst and a microbial community for microbial electrosynthesis. ChemSusChem 2021, 86, 763–777. [Google Scholar]
- Jourdin, L.; Sousa, J.; Stralen, N.; Strik, D.P.B.T.B. Techno-economic assessment of microbial electrosynthesis from CO2 and/or organics: An interdisciplinary roadmap towards future research and application. Appl. Energy 2020, 279, 115775. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Singh, R.; Bose, A. Microbial electron uptake in microbial electrosynthesis: A mini-review. J. Ind. Microbiol. Biotechnol. 2019, 46, 1419–1426. [Google Scholar] [CrossRef]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: Opportunities and challenges. Bioreousr. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, H.J.; Park, J.-M.; Lee, J.H.; Park, J.-S.; Shin, H.S.; Kim, Y.-H.; Min, J. Bacterial hydrogen production in recombinant Escherichia coli harboring a HupSL hydrogenase isolated from Rhodobacter sphaeroides under anaerobic dark culture. Int. J. Hydrogen Energy 2010, 35, 1112–1116. [Google Scholar] [CrossRef]
- Li, S.; Sakuntala, M.; Song, Y.E.; Heo, J.-O.; Kim, M.; Lee, S.Y.; Kim, M.-S.; Oh, Y.-K.; Kim, J.R. Photoautotrophic hydrogen production of Rhodobacter sphaeroides in a microbial electrosynthesis cell. Bioresour. Technol. 2021, 320, 124333. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.-L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef]
- Christodoulou, X.; Okoroafor, T.; Parry, S.; Velasquez-Orta, S.B. The use of carbon dioxide in microbial electrosynthesis: Advancements, sustainability and economic feasibility. J. CO2 Util. 2017, 18, 390–399. [Google Scholar] [CrossRef]
- Izadi, P.; Fontmorin, J.-M.; Godain, A.; Yu, E.H.; Head, I.M. Parameters influencing the development of highly conductive and efficient biofilm during microbial electrosynthesis: The importance of applied potential and inorganic carbon source. NPJ Biofilms Microbiomes 2020, 6, 40. [Google Scholar] [CrossRef]
- Aryal, N.; Halder, A.; Tremblay, P.-L.; Chi, Q.; Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 2016, 217, 117–122. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyebos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Min, J.; Lee, S.; Fitriana, H.N.; Kim, M.-S.; Park, G.W.; Lee, J.-S. Bioelectricity generation by Corynebacterium glutamicum with redox-hydrogel-modified carbon electrode. Appl. Sci. 2019, 9, 4251. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiao, S.; Fu, Q.; Kobayashi, H.; Zhang, L.; Liao, Q. Startup cathode potentials determine electron transfer behaviours of biocathodes catalyzing CO2 reduction to CH4 in microbial electrosynthesis. J. CO2 Util. 2020, 35, 169–175. [Google Scholar] [CrossRef]
- Navin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 2010, 1, e00103–e00110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajracharya, S.; Heine, A.; Benetton, X.D.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 2015, 195, 14–24. [Google Scholar] [CrossRef] [PubMed]
- McCully, A.L.; Spormann, A.M. Direct cathodic electron uptake coupled to sulfate reduction by Desulfovibrio ferrophilus IS5 biofilms. Environ. Microbiol. 2020, 22, 4794–4807. [Google Scholar] [CrossRef]
- Mao, J.; Won, S.W.; Yun, Y.-S. Development of poly(acrylic acid)-modified bacterial biomass as a high-performance biosorbent for removal of Cd(II) from aqueous solution. Ind. Eng. Chem. Res. 2013, 52, 6446–6452. [Google Scholar] [CrossRef]
- Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C 2019, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Maia, S.; Gomes, J.; Gomes, P.; Martins, M.C.L. Characterization of hLF1-11 immobilization onto chitosan ultrathin films, and its effects on antimicrobial activity. Acta Miomater. 2014, 10, 3513–3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.E.; Dhinojwala, A.; Moore, F.B.-G. Effect of substrate and bacterial zeta potential on adhesion of Mydobacterium smegmatis. Langmuir 2019, 35, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Anzai, J. Redox reactions of ferricyanide ions in layer-by-layer deposited polysaccharide films: A significant effect of the type of polycation in the films. Langmuir 2007, 23, 7378–7384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, Y.S.; Shin, W.-R.; Yu, J.; Lee, J.; Lee, S.; Kim, Y.-H.; Min, J. Non-photosynthetic CO2 bio-mitigation by Escherichia coli harbouring CBB genes. Green Chem. 2020, 22, 6889–6896. [Google Scholar] [CrossRef]
- Khan, N.E.; Myers, J.A.; Tuerk, A.L.; Curtis, W.R. A process economic assessment of hydrocarbon biofuels production using chemoautotrophic organisms. Bioresour. Technol. 2014, 172, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-Y.; Kim, Y.-H.; Min, J. CO2 reduction and organic compounds production by photosynthetic bacteria with surface displayed carbonic anhydrase and inducible expression of phosphoenolpyruvate carboxylate. Enzym. Microb. Technol. 2017, 96, 103–110. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, D.; Yan, X.; Zhou, Z.; Lee, J.K.; Yang, C. Network identification and flux quantification of glucose metabolism in Rhodobacter sphaeroides under photoheterotrophic H2-producing conditions. J. Bacteriol. 2012, 194, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Ukei, T.; Nevin, K.P.; Woodard, T.L.; Aklujkar, M.A.; Holmes, D.E.; Lovley, D.R. Construction of a Geobacter strain with exceptional growth on cathodes. Front. Microbiol. 2018, 9, 1512. [Google Scholar] [CrossRef] [Green Version]






| ζ-Potential (mV) | |
|---|---|
| Rhodobacter sphaeroides | −31.88 ± 3.31 |
| Bare graphite felt | −10.13 ± 2.72 |
| Graphite felt/chitosan | 1.81 ± 0.61 |
| Graphite felt/chitosan/carbodiimide | 1.74 ± 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitriana, H.N.; Lee, J.; Lee, S.; Moon, M.; Lee, Y.R.; Oh, Y.-K.; Park, M.; Lee, J.-S.; Song, J.; Lee, S.Y. Surface Modification of a Graphite Felt Cathode with Amide-Coupling Enhances the Electron Uptake of Rhodobacter sphaeroides. Appl. Sci. 2021, 11, 7585. https://doi.org/10.3390/app11167585
Fitriana HN, Lee J, Lee S, Moon M, Lee YR, Oh Y-K, Park M, Lee J-S, Song J, Lee SY. Surface Modification of a Graphite Felt Cathode with Amide-Coupling Enhances the Electron Uptake of Rhodobacter sphaeroides. Applied Sciences. 2021; 11(16):7585. https://doi.org/10.3390/app11167585
Chicago/Turabian StyleFitriana, Hana Nur, Jiye Lee, Sangmin Lee, Myounghoon Moon, Yu Rim Lee, You-Kwan Oh, Myeonghwa Park, Jin-Suk Lee, Jinju Song, and Soo Youn Lee. 2021. "Surface Modification of a Graphite Felt Cathode with Amide-Coupling Enhances the Electron Uptake of Rhodobacter sphaeroides" Applied Sciences 11, no. 16: 7585. https://doi.org/10.3390/app11167585