1. Introduction
Tissue engineering (TE) scaffolds temporarily provide the cells with a three-dimensional (3D) structure until the cells produce their own extracellular matrix (ECM) to replace the scaffold. Scaffolds should sustain cell growth and collagen production and provide guidance to the newly developed tissue [
1]. Ideally, scaffolds should also mimic the native 3D ECM structure to guide cell growth in a specific direction and be produced in a reproducible and inexpensive manner. The scaffold microstructure is characterised by its porosity, pore size, pore shape, interconnectivity and orientation. Each of these parameters influences the physical properties (e.g., mechanical properties and degradation rate) and biological properties (e.g., cell proliferation, cell differentiation, collagen production and angiogenesis), and ultimately control the growth of the new tissue [
2]. One of the main factors that influence the scaffold microstructure is the scaffold fabrication method, such as freeze drying, electrospinning or porogen leaching. Freeze drying is a fabrication technique that involves dissolving or suspending a material in a solvent, which is then frozen and sublimed, leaving a porous scaffold with a microstructure mirroring the ice crystals that formed during the freezing of the solution [
3]. Therefore, by controlling the ice crystal growth during the freezing of the solution, the microstructure of the scaffold can be designed and manufactured to mimic the ECM of native tissues, a process called ice-templating (also known as modified thermal induced phase separation).
The focus of this study was to utilise ice-templating based on freeze drying and custom-made moulds, in combination with airbrushing to fabricate polymer scaffolds mimicking the microstructure of soft tissues such as skin, oral mucosa and tendon. Furthermore, it is also important to use a biomaterial that is capable of showing tuneable physical properties in order to match its properties as close as possible to the target tissue. A promising material to be used as a soft tissue engineering scaffold is a biocompatible and biodegradable elastomer, poly(glycerol sebacate urethane) (PGSU). Pereira et al. [
4] showed that PGSU had tuneable mechanical properties and degradation rates by altering the molar ratio of the reactants, as well as excellent biological properties in vitro. They then proceeded to subcutaneously implant PGSU films in rat animal models for a 40-week period, examining the acute and chronic inflammatory response. No adverse reaction or any other complications were found over the 40-week period, and, at some time-points, the PGSU had less foreign body response compared to poly(lactic-co-glycolic acid) (PLGA), which is a Food and Drug Administration (FDA)-approved material for internal use. Frydrych et al. [
5] used freeze drying to produce PGSU scaffolds and found that by reducing the ratio of glycerol:hexamethylene diissocyanate (HDI), the degradation rate of the scaffolds became slower, and the mechanical properties changed. Subsequently, a study by Samourides et al. [
6] examined the control of the microstructure of PGSU scaffolds by altering the concentration of the PGSU pre-polymer in the freezing solution during fabrication. It was found that by increasing the pre-polymer concentration, the pore size became smaller, degradation rate was reduced and mechanical properties were enhanced. It was also demonstrated that the metabolic activity of fibroblast cells was not affected by the pore size, but the collagen production, angiogenesis and tissue ingrowth were significantly better for the PGSU scaffolds that had pore sizes around 28.2 μm when compared to the other scaffolds investigated [
6].
To mimic the architecture of soft tissues, it is necessary to understand their microstructure and function. Skin tissue functions as a protective barrier to separate the underlying tissue from the environment. It consists of three layers: the hypodermis, dermis and epidermis (epithelium), of which the latter two are separated by a basement membrane (BM) [
7]. Culturing a stratified epithelium, as found in the skin, requires an adhesive surface which is porous to nutrients but does not allow cell invasion to support the growth and differentiation of epithelial cells [
8]. Fibroblast cells found in the lamina propria or dermis support the adhesion of epithelial cells and require a porous scaffold with interconnected pores which mimic the ECM structures found in native tissue. The hypodermis is a loose connective tissue with large pore, capable of supporting the growth of large adipocyte cells. For skin tissue, a biomimetic scaffold should exhibit three layers, i.e., two porous layers with different pore sizes to support fibroblasts and adipocytes, and a BM-like layer on top to provide a surface to which epithelial cells can attach and grow without infiltrating the other two layers. Tendon tissue is comprised of a single tissue layer populated with tenocytes, but has an oriented microstructure in the load axis to connect a muscle with a bone and transfer the forces induced from the muscle to the bone to create motion [
9]. Therefore, the scaffold for tendon tissue should ideally have an oriented anisotropic, porous structure to allow tenocytes to penetrate, distribute and produce tendon tissue in a guided anisotropic structure.
Multilayer scaffolds were fabricated to mimic the multilayer structure of native tissues using various methods [
10,
11]. For example, silicon wafer moulds coated with a maltose-sacrificial layer were used to fabricate double-layer poly(glycerol sebacate) (PGS) porous scaffold with 50–250 μm pore size for the purpose of cardiac tissue engineering [
10]. It was found that the bilayer structure of the scaffolds provided a platform for cell delivery, promoted cell growth and had mechanical properties similar to the normal heart muscle. However, the disadvantage of the micromoulding fabrication technique is that it requires multiple steps to prepare the moulds, involves a sacrificial layer to allow the detachment of the cast polymer and can only produce thin scaffolds, approximately 150 μm thick, which limits their scalability and application. In another study, casting, moulding and freeze drying techniques were used to develop a natural triple-layered vascular graft made out of collagen type I, fibrils and elastin fibres for vascular tissue engineering [
11]. Three tubular moulds that had different sizes were used to build the scaffold layer by layer, and it was found that the scaffolds exhibited suitable morphologies and properties for vascular tissue engineering.
In addition, anisotropic monolayer scaffolds were also manufactured for tissues that require anisotropy [
12]. The advantage of having oriented pore architecture is that it can mimic the natural in vivo ECM of those tissues which require alignment—for example, tendon and skeletal muscle. Successful attempts to fabricate scaffolds with anisotropic pore architecture by using freeze drying and custom-made moulds that either produced temperature gradient due to difference in the thermal conductivity between the mould materials [
13,
14,
15,
16], or submerged the solvent into a cold bath at a constant rate [
17], were reported using various biomaterials, such as collagen, gelatin, poly(vinyl alcohol) and PLGA. The custom-made moulds were comprised of either a metal base and Perspex walls [
13], polytetrafluoroethylene (PTFE) mould placed on a copper cold finger [
14], metal base with polyethylene walls [
15] or plastic tubes that had their walls further isolated with Styrofoam [
16]. These moulds induced a temperature gradient during the freezing of the polymer solution, and after freeze drying, the resulting scaffolds exhibited anisotropic microstructures but, in some cases, the scaffold microstructures were characterised by a dense non-oriented layer at the bottom due to a higher cooling rate [
13,
14]. Generally, the scaffolds showed optimised mechanical properties in the direction of the pores, and the new ECM produced was aligned with the pores.
While multilayer and anisotropic monolayer scaffolds were fabricated before and showed promising results in multiple TE applications, the fabrication techniques used usually produce small-size scaffolds, require multiple steps and a long fabrication time, and have limited control between layer microfeatures (pore size, pore orientation, porosity etc.). This study aims to demonstrate the techniques of ice-templating and airbrushing that can be combined to fabricate large, complex, hierarchical structures in a controlled manner for soft TE. PGSU scaffolds were fabricated as two-layer, three-layer and anisotropic single layer. Ice-templating via freeze drying and custom-made moulds was utilised to produce large 3D porous scaffolds with controlled pore orientation and, in combination with airbrushing, a thin non-porous film could be deposited on top of the porous scaffold to mimic tissue structures that intrinsically exhibit a BM. Scanning electron microscopy (SEM) was used to characterise the microstructure of the PGSU scaffolds. To investigate the potential applications of the anisotropic PGSU scaffolds, a range of scaffolds were fabricated with multiple pre-polymer concentrations and glycerol:HDI molar ratios and tensile testing was used to evaluate the mechanical properties.
3. Results
The chemical structure of the PGSU synthesised was previously confirmed using Fourier-transform infrared spectroscopy [
6].
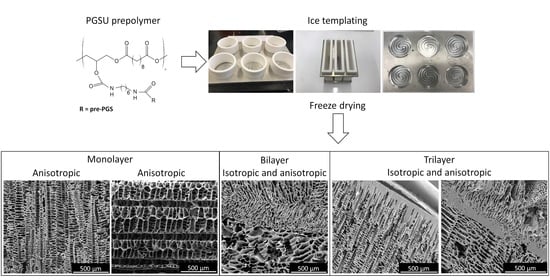
Figure 1 shows the SEM images from the PGSU-perpendicular scaffolds fabricated using mould-perpendicular. As expected, this scaffold had a unidirectional pore structure, moving from the bottom to top (perpendicular) forming porous channels (cross-section view), which interconnect with the top part of the scaffold. The pore size of the PGSU-perpendicular is significantly different between its cross-section and top section, with pore sizes of 34.1 ± 9.6 μm and 40.3 ± 4.6 μm, respectively.
The SEM images of the PGSU-parallel scaffold are shown in
Figure 2. As described before, the scaffold was rotated by 90° before investigation. The resulting scaffold had a rectangular parallelepiped shape and the pore direction was unidirectional from the left to right. A uniform structure is observed with porous channels formed along the horizontal axis. When it was examined for its cross-section, open pores were found throughout the scaffold. The uniformity of the pore structure is also demonstrated from the pore size measurements. The PGSU-parallel scaffold showed no significant difference in pore size between the top, cross- and bottom sections, and its pore sizes ranged from 67.8 ± 20.0 μm to 78.8 ± 24.2 μm.
Figure 3 illustrates the microstructure of the PGSU-bilayer scaffolds. In this case, the top layer was made from PGSU-15% and bottom layer from PGSU-10%, thus there is a difference in pore structure when it is observed from the cross-section. The bilayer scaffold shows both isotropic and anisotropic structures. It is clear where the layers meet and a good attachment between them is also evident. The pore structure on the surface is similar to when the scaffold was being imaged from its top and bottom section. The pore sizes of the PGSU-bilayer were measured, and multiple pore sizes were found depending on the layer and the section. Considering each layer individually, there was a significant difference between the pore size of the top layer (PGSU-15%) when compared with that of its cross-section. A similar observation was found from the bottom layer (PGSU-10%). There was also a significant difference between the pore size of the different layers, especially in the cross-section. The pore size for the PGSU-15% layer was 26.2 ± 4.9 μm and the pore size for the PGSU-10% layer was 74.0 ± 8.4 μm, which is an approximately 2-fold difference.
The last scaffold that was fabricated was PGSU-trilayer and the results are shown in
Figure 4. In this case, the top layer acts as a BM, which is a thin non-porous film (~92 μm thick) and, at the same time, the bottom section has an open pore structure. The bottom two layers of the PGSU-trilayer scaffold exhibited both isotropic and anisotropic microstructures, similar to the case in the PGSU-bilayer. The connection between all three layers is also shown with red arrows, which confirms that one solid scaffold can be fabricated from three different layers, that can be distinguished by their difference in pore structure and porosities. As expected, the pore size was significantly different between the bottom two layers of the scaffold, with the upper layer showing a pore size of 20.3 ± 5.0 μm and the lower layer showing a pore size of 49.2 ± 10.8 μm. This demonstrates that, by changing the polymer concentration, different pore sizes can be achieved, and that by attaching these layers on top of each other, a single scaffold can exhibit multiple pore sizes.
The pore sizes of all the scaffolds described above are summarised in
Figure 5 for ease of comparison. A summary of the scaffolds fabricated in this work is also outlined in
Table 2. Multiple pore structures, pore orientations and pore sizes were obtained using PGSU, freeze drying with custom-made moulds and airbrushing.
The tensile properties of the PGSU-parallel scaffolds at different pre-polymer concentrations and glycerol:HDI molar ratios were measured and the results are shown in
Figure 6. It can be noted that, in almost all cases, there was a significant difference between the ultimate tensile strength (UTS) and Young’s modulus (E) of the samples but not the elongation at break. This signifies that the glycerol:HDI molar ratio and the pre-polymer concentration do not affect the elongation at break, except for the scaffolds with 20% pre-polymer concentration, which were found to be lower than the rest and, in one case, a statistically significant difference was found.. The scaffolds exhibited a UTS ranging from 0.25 ± 0.04 MPa to 1.91 ± 0.10 MPa and an E ranging from 0.33 ± 0.05 MPa to 1.97 ± 0.26 MPa. The biggest difference was found when comparing the scaffolds with glycerol:HDI molar ratio of 1:0.8 against the case with the ratio of 1:1.0. The UTS and E both increased by approximately 3-fold, while the elongation at break remained very similar. This is an advantage of the PGSU scaffolds developed here, because they have the potential to be used for multiple tissues with varying mechanical properties (e.g., tendon, skin, ligaments and mucosa) [
20,
21].
4. Discussion
This study aimed to fabricate PGSU scaffolds using freeze drying together with custom-made ice-templating moulds, with or without the airbrushing technique, to produce microstructures suitable for different tissue engineering applications. Three model microstructures (anisotropic, bilayer and trilayer with different porous structures) were fabricated from PGSU scaffolds using different mould designs and different freeze drying techniques, along with the airbrushing technique.
The objective of ice-templating is to control the microstructure of the scaffold by controlling the ice crystal formation. Ice-templating is not a new technique, and there are numerous studies that used it to fabricate anisotropic scaffolds for a range of applications, including cartilage, skeletal muscle, tendon and neuron [
16,
22,
23]. To characterise the ice-templating and mould technology, we investigated the thermal profiles of the in-house moulds (
Figure S3). These moulds were designed with the intention of allowing heat to enter the polymer solution in an isotropic or anisotropic manner, dependent on the scaffold that was produced. These different moulds were expected to enable (i) the polymer solution to freeze relatively homogeneously, with heat distributed from multiple directions within the polymer solution to create an isotropic (random) structure; (ii) the polymer solution to freeze from only one direction, with heat distributed from one direction to create a uniform anisotropic (oriented) structure. When mould-isotropic was used to fabricate the bilayer and trilayer scaffolds, it was found that the scaffolds also showed some anisotropic microstructure in addition to the isotropic microstructure. The presence of the anisotropic structure in the scaffold, while mould-isotropic was used, was also found in some other freeze-dried scaffolds [
24], presumably in part due to 1,4-dioxane having two crystalline phases (phase I existing between 5–12 °C and phase II between −140–5 °C); in phase I, the 1,4-dioxane ice crystals grow upwards, and in phase II they begin to form branches on the already formed ice crystals [
25]. In addition, the polymer solution (with a total height of 10 mm) in mould-isotropic froze at its bottom (0 mm height) and middle (5 mm height) with 11 min difference, shown in
Figure S3A (Supplementary Content) which induced a modest anisotropic heat transfer and, therefore, some anisotropic structure. While the height of the polymer solution used to create each porous layer of bilayer and trilayer scaffolds was only 5 mm, the modest anisotropic heat transfer should also have been presented during freezing, leading to some anisotropic microstructures, in particular in the lower region of the scaffolds. The solution in the upper region froze in a more isotropic manner, resulting in a more isotropic microstructure (in the second layer for PGSU-bilayer (
Figure 3) and mid layer for PGSU-trilayer (
Figure 4). When the walls of the mould were insulated using PTFE (low thermal conductivity), the polymer solution froze from the bottom to the top, resulting in an anisotropic heat transfer and, therefore, anisotropic scaffold microstructure, as in
Figure 1 and
Figure 2. The term “isotropic” means that the scaffold exhibits the same structure and properties (mechanical properties, permeability, etc.) when viewed from any direction, while “anisotropic” distributes the structure and properties to a specific direction (along the pore alignment).
The PGSU-parallel scaffolds were prepared with multiple conditions, aiming to quantify the range of mechanical properties that such scaffolds can achieve. The mechanical properties of a scaffold depend on several factors, such as the properties of the polymer, and the pore size, porosity and pore orientation of the scaffolds [
5,
6,
26]. In previous studies, it was found that PGSU scaffolds can exhibit different properties by either changing the glycerol:HDI molar ratio or changing the pre-polymer concentration. Frydrych and Chen [
5] fabricated PGSU scaffolds for soft TE and characterised the effect that the glycerol:HDI molar ratio has on the mechanical properties of the scaffold. It was found that by reducing the glycerol:HDI ratio from 1:0.4 to 1:0.6, the scaffolds exhibited a UTS between 18 and 22 kPa, E between 30 and 40 kPa and elongation at break between 49 and 82%. Another study from us [
6] showed that by increasing the pre-polymer concentration of the PGSU solution, the fabricated scaffolds exhibited more uniform microstructures, with a UTS and E between 0.05-0.86 MPa and 0.05–0.65 MPa, respectively. In this work, both variables, glycerol:HDI molar ratio and pre-polymer concentration, were combined, to further characterise the mechanical properties that can be achieved by PGSU scaffolds. It was found that the UTS (0.25–1.91 MPa) and E (0.33–1.97 MPa) of this study were 1–6 times higher than the scaffolds fabricated by Samourides et al. [
6] and 10–85 times higher from the scaffolds fabricated by Frydrych and Chen [
5]. This significant difference in mechanical strength is attributed to the fact that the HDI:glycerol molar ratio and pre-polymer concentration in this study were higher, and that the anisotropic microstructure made the scaffolds exhibit higher strength and more resistance to deformation over the axis of elongation. This means that the PGSU scaffolds can be fabricated for more tissues, which require a better mechanical support in a single direction, such as skeletal muscle and tendon tissues.
Multilayer ECM structures are usually found when a tissue is composed from multiple cell types and functionalities. Such tissues could be skin and oral mucosa [
27]. These tissues have different tissue layers and are separated by a BM, which functions by separating the tissue layers while allowing gas/nutrient exchange and cell communication. Research in fabricating multilayer scaffolds has been successful in the past, using multiple materials (collagen, poly(ε-caprolactone), PLGA) and fabrication techniques (porogen leaching, electrospinning, freeze drying, 3D printing) [
28,
29,
30]. The aim of all these studies, including this work, was to mimic the complex hierarchical multilayer characteristics of native ECM, which has been shown to guide tissue development and stem cell differentiation [
29,
31]. Tissue development may be enhanced by varying the pore size, porosity and mechanical properties. For example, in cartilage tissue engineering a bilayer scaffold was fabricated with PLGA and collagen [
32]. These scaffolds were then seeded with mesenchymal stem cells (MSCs) and, 4 months after implantation into a 1-year-old beagle, osteochondral tissue was regenerated with cartilage- and bone-like tissues in each respective layer [
32]. Here, we propose a similar approach; however, to fabricate the hierarchical multilayer scaffolds, two fabrication techniques were combined, freeze drying and airbrushing, using the same polymer. The combination of these techniques allowed for the design of novel PGSU scaffolds that either exhibited a thin non-porous layer (BM-like structure) or multiple porous layers with different pore structures, sizes and porosities. The difference in pore structure, size and porosity is there to provide the appropriate structure either for MSCs to differentiate to localised specific cells or for those specific cells to be seeded individually and reside within a biomimetic environment. The BM-like structure, as a third layer of the scaffold, is there to restrict the epithelial cells from penetrating into the porous section of the scaffold, where fibroblasts are supposed to reside.
While further investigation is required, the present study demonstrates a relatively simple method to fabricate synthetic scaffolds with hierarchical architecture using the same polymer for skin, tendon tissue engineering and possible other tissue engineering applications. Where desirable, different polymers with other physical and biological properties may also be used to fabricate the hierarchical and controlled microstructures demonstrated in this work. With some improvements in the fabrication techniques and adjustment of the cell co-culture methods to suit this study’s microstructures, such scaffolds have the potential to be used as a skin or an oral mucosa tissue equivalent to measure toxicity, drug delivery and to model diseases (similar to [
33,
34,
35]), as well as for the purpose of regenerating skin or oral mucosa tissue. PGSU, in terms of scalability, can be synthesised and fabricated into large quantities at a low cost; the scaffold is reproducible, and with controlled chemical synthesis and freeze drying, the reproducibility could be high. Furthermore, there are multiple ways to alter both the mechanical properties and degradation rates of the polymer scaffolds (e.g., by changing the glycerol:HDI molar ratio and/or the pre-polymer concentration in the freeze drying solution).