Heavy Metal Extraction under Environmentally Relevant Conditions Using 3-Hydroxy-2-Naphthoate- Based Ionic Liquids: Extraction Capabilities vs. Acute Algal Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Extraction Experiments
2.4. Growth Inhibition Assays
2.5. Chlorophyll Fluorescence
3. Results and Discussion
3.1. Extraction Experiments
3.1.1. Extraction of Hg
3.1.2. Extraction of Cu, Ag, Cd, Hg and Pb from 20 µg L−1 Feed Solutions
3.2. Leaching
3.3. Acute Toxicity Assays
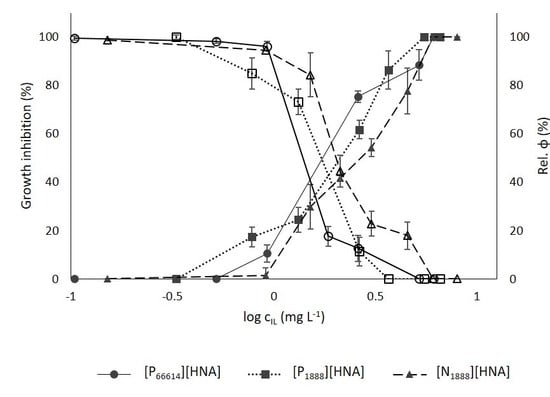
3.3.1. Growth Inhibition
3.3.2. Chlorophyll Fluorescence
3.3.3. Ionic Liquid vs. Heavy Metal Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.; Chen, B.; Koo, Y.-M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.-J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef]
- Feldmann, C.; Ruck, M. Ionic Liquids—Designer Solvents for the Synthesis of New Compounds and Functional Materials. Z. Anorg. Allg. Chem. 2017, 643, 2. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhang, Z.; Sun, X.-G.; Hu, Y.-S.; Xing, H.; Dai, S. Ionic liquids and derived materials for lithium and sodium batteries. Chem. Soc. Rev. 2018, 47, 2020–2064. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S.-J. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Shamshima, J.L.; Berton, P.; Wang, H.; Zhou, X.; Gurau, G.; Rodgers, R.D. Ionic Liquids in Pharmaceutical Industry. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 539–577. [Google Scholar]
- Stojanovic, A.; Keppler, B.K. Ionic Liquids as Extracting Agents for Heavy Metals. Sep. Sci. Technol. 2012, 47, 189–203. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J., Jr.; Rogers, R.D. Task-Specific Ionic Liquids for the Extraction of Metal Ions from Aqueous Solutions. Chem. Commun. 2001, 1, 135–136. [Google Scholar] [CrossRef]
- Fischer, L.; Falta, T.; Koellensperger, G.; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B.K.; Hann, S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water. Water Res. 2011, 45, 4601–4614. [Google Scholar] [CrossRef]
- Leyma, R.; Platzer, S.; Jirsa, F.; Kandioller, W.; Krachler, R.; Keppler, B.K. Novel thiosalicylate-based ionic liquids for heavy metal extractions. J. Hazard. Mater. 2016, 314, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platzer, S.; Leyma, R.; Wolske, S.; Kandioller, W.; Heid, E.; Schröder, C.; Schagerl, M.; Krachler, R.; Jirsa, F.; Keppler, B.K. Thioglycolate-based task-specific ionic liquids: Metal extraction abilities vs. acute algal toxicity. J. Hazard. Mater. 2017, 340, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kogelnig, D.; Stojanovic, A.; Galanski, M.; Groessl, M.; Jirsa, F.; Krachler, R.; Keppler, B.K.; Krachler, M. Greener synthesis of new ammonium ionic liquids and their potential as extracting agents. Tetrahedron Lett. 2008, 49, 2782–2785. [Google Scholar] [CrossRef]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; Macfarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Dietz, M.L.; A Dzielawa, J. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: Implications for the ’greenness’ of ionic liquids as diluents in liquid-liquid extraction. Chem. Commun. 2001, 20, 2124–2125. [Google Scholar] [CrossRef]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Guillon, E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: Cation exchange versus ion-pair extraction. Sep. Purif. Technol. 2013, 107, 172–178. [Google Scholar] [CrossRef]
- Dietz, M.L.; Stepinski, D.C. A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): implications for the “greenness” of RTILs as extraction solvents. Green Chem. 2005, 7, 747. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Nancharaiah, Y.; Venugopalan, V. Long alkyl-chain imidazolium ionic liquids: Antibiofilm activity against phototrophic biofilms. Colloids Surfaces B Biointerfaces 2017, 155, 487–496. [Google Scholar] [CrossRef]
- Scammells, P.J.; Scott, J.L.; Singer, R.D. Ionic Liquids: The Neglected Issues. Aust. J. Chem. 2005, 58, 155–169. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(Cyto)Activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Papaiconomou, N.; Lee, J.-M.; Salminen, J.; Clark, D.S.; Prausnitz, J.M. In vitrocytotoxicities of ionic liquids: Effect of cation rings, functional groups, and anions. Environ. Toxicol. 2009, 24, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-W.; Jeon, Y.-C.; Pham, T.P.T.; Vijayaraghavan, K.; Yun, Y.-S. The ecotoxicity of ionic liquids and traditional organic solvents on microalga Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2008, 71, 166–171. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Thiele, K.; Juffernholz, T.; Arning, J.; Ranke, J.; Welz-Biermann, U.; Jastorff, B. The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco)toxicological test battery. Green Chem. 2007, 9, 1198. [Google Scholar] [CrossRef]
- Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Novel 3-Hydroxy-2-Naphthoate-Based Task-Specific Ionic Liquids for an Efficient Extraction of Heavy Metals. Front. Chem. 2018, 6, 172. [Google Scholar] [CrossRef]
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence-a Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA) Bioenergy 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Juneau, P.; Dewez, D.; Matsui, S.; Kim, S.-G.; Popovic, R. Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere 2001, 45, 589–598. [Google Scholar] [CrossRef]
- Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids. Molecules 2018, 23, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germani, R.; Mancini, M.V.; Savelli, G.; Spreti, N. Mercury extraction by ionic liquids: temperature and alkyl chain length effect. Tetrahedron Lett. 2007, 48, 1767–1769. [Google Scholar] [CrossRef]
- EU. Directive 2000/60/Ec of the European Parliament and the Council Establishing a Framework for the Community in the Field of Water Policy; European Union: Brussels, Belgium, 2000. [Google Scholar]
- Republic of Austria. Verordnung des Bundesministers Für Soziale Sicherheit Und Generationen Über Die Qualität Von Wasser Für Den Menschlichen Gebrauch (Trinkwasserverordnung—TWV). In BGBl. II Nr. 304/2001; Bundeskanzleramt Österreich: Vienna, Austria, 2001. [Google Scholar]
- EU. Directive 2008/105/Ec of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/Eec, 83/513/Eec, 84/156/Eec, 84/491/Eec, 86/280/Eec and Amending Directive 2000/60/Ec of the European Parliament and of the Council; European Parliament: European Union: Brussels, Belgium, 2008. [Google Scholar]
- Rodrigues, A.; Jesus, F.; Fernandes, M.; Morgado, F.; Soares, A.M.; Abreu, S. Mercury Toxicity to Freshwater Organisms: Extrapolation Using Species Sensitivity Distribution. Bull. Environ. Contam. Toxicol. 2013, 91, 191–196. [Google Scholar] [CrossRef]
- Wood, C.M. Silver. In Homeostasis and Toxicology of Non-Essential Metals; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ratte, H.T. Bioaccumulation and Toxicity of Silver Compounds: A Review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Schmittschmitt, J.P.; Shaw, J.R.; Birge, W.J. Effects of Silver on Green Algae and Prospects for Trophic Transfer. In Proceedings of the 4th International Conference Proceedings: Transport, Fate and Effects of Silver in the Environment, Madison, WI, USA, 25–28 August 1996. [Google Scholar]
- Heijerick, D.G.; Bossuyt, B.T.; De Schamphelaere, K.A.; Indeherberg, M.; Mingazzini, M.; Janssen, C.R. Effect of Varying Physicochemistry of European Surface Waters on the Copper Toxicity to the Green Alga Pseudokirchneriella subcapitata. Ecotoxicology 2005, 14, 661–670. [Google Scholar]
- Janssen, C.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M. Metal extraction to ionic liquids: the relationship between structure, mechanism and application. Int. Rev. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621. [Google Scholar] [CrossRef]
- Park, S.-M.; Choi, J.; Nam, T.-G.; Ku, J.-M.; Jeong, K. Anti-diabetic effect of 3-hydroxy-2-naphthoic acid, an endoplasmic reticulum stress-reducing chemical chaperone. Eur. J. Pharmacol. 2016, 779, 157–167. [Google Scholar] [CrossRef]
- Ma, J.-M.; Cai, L.-L.; Zhang, B.-J.; Hu, L.-W.; Li, X.-Y.; Wang, J. Acute toxicity and effects of 1-alkyl-3-methylimidazolium bromide ionic liquids on green algae. Ecotoxicol. Environ. Saf. 2010, 73, 1465–1469. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Chen, C.; Du, S.; Dong, Y. Effects of imidazolium chloride ionic liquids and their toxicity to Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2015, 122, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, H.; Wang, S.; Chen, J.; Xia, Y. The toxicity of ionic liquid 1-decylpyridinium bromide to the algae Scenedesmus obliquus: Growth inhibition, phototoxicity, and oxidative stress. Sci. Total Environ. 2018, 622–623, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- UN. Globally Harmonized System of Classification and Labelling of Chemicals (Ghs); United Nations: New York, NY, USA; Geneva, Switzerland, 2011. [Google Scholar]
- Kumar, K.S.K.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Fai, P.B.; Grant, A.; Reid, B. Chlorophyll a fluorescence as a biomarker for rapid toxicity assessment. Environ. Toxicol. Chem. 2007, 26, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, X.; Zhou, Q.; Dong, K.; Yao, H.; Zhang, S.-J. Biodegradable Naphthenic Acid Ionic Liquids: Synthesis, Characterization, and Quantitative Structure-Biodegradation Relationship. Chem. A Eur. J. 2008, 14, 11174–11182. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.; Fudała, J.; Strzelecka-Jastrząb, E.; Hlawiczka, S.; Panasiuk, D.; Nitter, S.; Pregger, T.; Pfeiffer, H.; Friedrich, R. Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmospheric Environ. 2007, 41, 8557–8566. [Google Scholar] [CrossRef]
- Vasseur, P.; Pandard, P.; Burnel, D. Influence of Some Experimental Factors on Metal Toxicity to Selenastrum capricornutum. Toxic. Assess. 1988, 3, 331–343. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, K.C. Optimization and Performance Evaluation of the Continuous Algal Toxicity Test. Environ. Toxicol. Chem. 1997, 16, 1337–1344. [Google Scholar] [CrossRef]
- Lin, J.-H.; Kao, W.-C.; Tsai, K.-P.; Chen, C.-Y. A novel algal toxicity testing technique for assessing the toxicity of both metallic and organic toxicants. Water Res. 2005, 39, 1869–1877. [Google Scholar] [CrossRef]
- Horvatic, J.; Persic, V.; Pavlic, Z.; Stjepanovic, B.; Has-Schön, E. Toxicity of Metals on the Growth of Raphidocelis Subcapitata and Chlorella Kessleri Using Microplate Assays. Fresenius Environ. Bull. 2007, 16, 826. [Google Scholar]
- Monteiro, C.; Caldas-Fonseca, S.; Castro, P.; Malcata, F.X. Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. Environ. Biol. Fishes 2010, 23, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Juneau, A.E.B.P.; Juneau, P.; El Berdey, A.; Popović, R. PAM Fluorometry in the Determination of the Sensitivity of Chlorella vulgaris, Selenastrum capricornutum, and Chlamydomonas reinhardtii to Copper. Arch. Environ. Contam. Toxicol. 2002, 42, 155–164. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Pirkwieser, P.; Leyma, R.; Kandioller, W.; Krachler, R.; Keppler, B.K.; Jirsa, F.; Moreno, C. Solvent bar micro-extraction for greener application of task specific ionic liquids in multi-elemental extraction. J. Clean. Prod. 2018, 201, 22–27. [Google Scholar] [CrossRef]



| pH | El. Cond. | Na+ | K+ | Mg2+ | Ca2+ | Cl- | SO42- |
|---|---|---|---|---|---|---|---|
| 7.22 | 846 (µS cm−1) | 14.2 | 1.8 | 39.5 | 95 | 23 | 221 |
| Residual concentration mean ± SD (µg L−1) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | IL | Cu | Ag | Cd | Hg | Pb | |||||||||||||||
| Pure waterpH 3.5 | [P66614][HNA] | 16.2 | ± | 0.5 | (21.3) | < 0.03 | (> 99) | 7.5 | ± | 0.3 | (64.4) | < 0.1 | (> 99) | 17.7±0.1 | (< 5) | ||||||
| [P1888][HNA] | 7.8 | ± | 2.3 | (62.4) | 13.2 | ± | 0.1 | (14.3) | 18.5 | ± | 1.0 | (13.3) | 1.7 | ± | 0.2 | (91.3) | 4.3 | ± | 0.1 | (75.6) | |
| [N1888][HNA] | 2.2 | ± | 0.8 | (89.1) | 3.6 | ± | 0.2 | (76.1) | 14.8 | ± | 1.3 | (30.4) | 2.6 | ± | 0.4 | (86.7) | 1.2 | ± | 0.6 | (93.0) | |
| Pure waterpH 8.0 | [P66614][HNA] | 10.5±1.8 | (< 5) | < 0.03 | (> 99) | 6.5 | ± | 0.9 | (37.3) | < 0.1 | (> 99) | n/a | |||||||||
| [P1888][HNA] | 6.6 | ± | 0.8 | (36.1) | 7.0 | ± | 0.9 | (33.1) | 9.3 | ± | 0.2 | (10.5) | 2.4 | ± | 0.2 | (87.8) | n/a | ||||
| [N1888][HNA] | 4.2 | ± | 0.7 | (59.7) | 6.8 | ± | 0.5 | (35.0) | 11.7±0.8 | (< 5) | 2.9 | ± | 1.2 | (85.4) | n/a | ||||||
| Mineral water | [P66614][HNA] | 1.3 | ± | 0.4 | (90.4) | < 0.03 | (> 99) | 12.7 | ± | 0.1 | (35.4) | < 0.1 | (> 99) | 6.0 | ± | 1.2 | (28.2) | ||||
| [P1888][HNA] | 1.1 | ± | 0.2 | (90.8) | 3.5 | ± | 0.3 | (75.3) | 16.0 | ± | 0.9 | (18.8) | 6.4 | ± | 0.1 | (68.0) | 4.5 | ± | 0.6 | (45.6) | |
| [N1888][HNA] | 0.8 | ± | 0.3 | (93.3) | 2.3 | ± | 0.7 | (83.6) | 13.5 | ± | 0.7 | (31.3) | 6.7 | ± | 0.2 | (66.3) | 5.2 | ± | 1.2 | (38.3) | |
| Leaching ± SD (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| Sample | [P66614][HNA] | [P1888][HNA] | [N1888][HNA] | |||
| Cu | ||||||
| Pure water pH 3.5 | 76.7 ± 1.1 | (0.38) | 24.7 ± 2.0 | (0.12) | 139.3 ± 23.8 | (0.70) |
| Pure water pH 8.0 | 75.9 ± 0.9 | (0.38) | 25.7 ± 2.3 | (0.13) | 136.8 ± 30.7 | (0.68) |
| Mineral water | 62.9 ± 1.1 | (0.31) | 27.6 ± 3.8 | (0.14) | 78.6 ± 6.0 | (0.39) |
| Ag | ||||||
| Pure water pH 3.5 | 75.0 ± 2.4 | (0.38) | 25.3 ± 0.4 | (0.13) | 168.1 ± 5.7 | (0.83) |
| Pure water pH 8.0 | 80.3 ± 0.7 | (0.40) | 23.2 ± 0.9 | (0.12) | 132.4 ± 9.8 | (0.66) |
| Mineral water | 74.4 ± 1.6 | (0.38) | 24.8 ± 7.9 | (0.12) | 99.8 ± 9.3 | (0.50) |
| Cd | ||||||
| Pure water pH 3.5 | 83.7 ± 2.5 | (0.42) | 25.6 ± 1.5 | (0.13) | 97.4 ± 6.1 | (0.48) |
| Pure water pH 8.0 | 78.6 ± 2.5 | (0.39) | 25.4 ± 1.2 | (0.24) | 80.4 ± 10.4 | (0.39) |
| Mineral water | 84.3 ± 0.5 | (0.42) | 43.5 ± 3.2 | (0.33) | 81.8 ± 6.0 | (0.35) |
| Hg | ||||||
| Pure water pH 3.5 | 80.2 ± 1.6 | (0.40) | 25.6 ± 2.0 | (0.13) | 137.1 ± 15.0 | (0.68) |
| Pure water pH 8.0 | 78.4 ± 0.6 | (0.39) | 24.8 ± 0.4 | (0.12) | 87.5 ± 2.3 | (0.44) |
| Mineral water | 65.4 ± 0.8 | (0.33) | 27.6 ± 2.4 | (0.14) | 80.6 ± 6.0 | (0.40) |
| Pb | ||||||
| Pure water pH 3.5 | 73.2 ± 8.0 | (0.36) | 25.4 ± 2.4 | (0.13) | 76.7 ± 2.7 | (0.38) |
| Pure water pH 8.0 | 74.6 ± 1.7 | (0.37) | 28.5 ± 1.5 | (0.14) | 107.1 ± 12.5 | (0.53) |
| Mineral water | 63.6 ± 1.2 | (0.32) | 27.3 ± 0.2 | (0.14) | 110.8 ± 4.8 | (0.55) |
| Ionic Liquid | MW [g mol−1] | Growth inhibition | Max. PSII quantum yield | ||
|---|---|---|---|---|---|
| T. obliquus EC50± SD | R. subcapitata EC50± SD | T. obliquus EC50± SD | R. subcapitata EC50± SD | ||
| [P66614][HNA] | 671.03 | 1.76 ± 0.17 | 0.47 ± 0.01 | 1.81 ± 0.03 | 0.52 ± 0.05 |
| [P1888][HNA] | 572.86 | 2.61 ± 0.06 | 0.39 ± 0.05 | 1.86 ± 0.06 | 0.13 ± 0.03 |
| [N1888][HNA] | 555.88 | 2.68 ± 0.44 | 0.28 ± 0.01 | 2.04 ± 0.22 | 0.24 ± 0.06 |
| [N1888][C6SAc] * | 543.98 | 0.93 ± 0.16 | 0.05 ± 0.01 | n/a | n/a |
| [P1888][C6SAc] * | 560.95 | 8.96 ± 0.43 | 0.04 ± 0.01 | n/a | n/a |
| [N1888][Cl] * | 404.16 | 0.30 ± 0.03 | 0.07 ± 0.01 | n/a | n/a |
| [P66614][Cl] * | 519.31 | 0.39 ± 0.02 | 0.10 ± 0.01 | n/a | n/a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirkwieser, P.; López-López, J.A.; Schagerl, M.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Heavy Metal Extraction under Environmentally Relevant Conditions Using 3-Hydroxy-2-Naphthoate- Based Ionic Liquids: Extraction Capabilities vs. Acute Algal Toxicity. Appl. Sci. 2020, 10, 3157. https://doi.org/10.3390/app10093157
Pirkwieser P, López-López JA, Schagerl M, Kandioller W, Keppler BK, Moreno C, Jirsa F. Heavy Metal Extraction under Environmentally Relevant Conditions Using 3-Hydroxy-2-Naphthoate- Based Ionic Liquids: Extraction Capabilities vs. Acute Algal Toxicity. Applied Sciences. 2020; 10(9):3157. https://doi.org/10.3390/app10093157
Chicago/Turabian StylePirkwieser, Philip, José A. López-López, Michael Schagerl, Wolfgang Kandioller, Bernhard K. Keppler, Carlos Moreno, and Franz Jirsa. 2020. "Heavy Metal Extraction under Environmentally Relevant Conditions Using 3-Hydroxy-2-Naphthoate- Based Ionic Liquids: Extraction Capabilities vs. Acute Algal Toxicity" Applied Sciences 10, no. 9: 3157. https://doi.org/10.3390/app10093157