Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
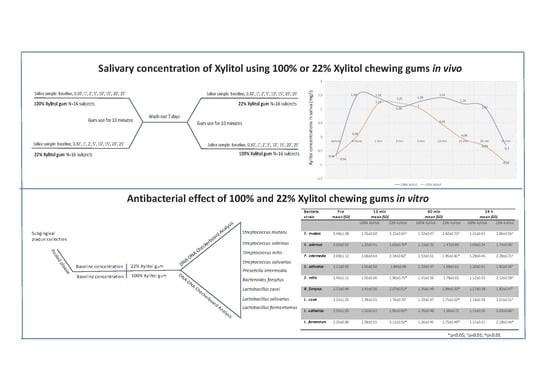
2.2. Xylitol Concentration In Saliva
2.2.1. Sample Size
2.2.2. Samples Collection and Measurements of the Concentration of Xylitol in Saliva
2.3. Antibacterial Activity of Xylitol
2.3.1. Methods
2.3.2. Specimen Preparation
2.3.3. Microbiological Analysis
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Chi, D.L.; Scott, J.M. Added Sugar and Dental Caries in Children: A Scientific Update and Future Steps. Dent. Clin. 2019, 63, 17–33. [Google Scholar]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderling, E.M. Xylitol, mutans streptococci, and dental plaque. Adv. Dent. Res. 2009, 21, 74–78. [Google Scholar] [CrossRef]
- Campus, G.; Cagetti, M.G.; Sale, S.; Petruzzi, M.; Solinas, G.; Strohmenger, L.; Lingström, P. Six months of high-dose xylitol in high-risk caries subjects--a 2-year randomised, clinical trial. Clin. Oral. Investig. 2013, 17, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanzer, J.M.; Thompson, A.; Wen, Z.T.; Burne, R.A. Streptococcus mutans: Fructose Transport, Xylitol Resistance, and Virulence. J. Dent. Res. 2006, 85, 369–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedy, C.A.; Milgrom, P.; Ly, K.A.; Rothen, M.; Mueller, G.; Hagstrom, M.K. A surrogate method for comparison analysis of salivary concentrations of Xylitol-containing products. BMC Oral Health 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiainen, T.; Renko, M.; Kontiokari, T.; Uhari, M. Xylitol concentrations in the saliva of children after chewing xylitol gum or consuming a xylitol mixture. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 53–55. [Google Scholar] [CrossRef]
- Lif Holgerson, P.; Stecksén-Blicks, C.; Sjostrom, I.; Oberg, M.; Twetman, S. Xylitol concentrations in saliva and dental plaque after use of various xylitol-containing products. Caries Res. 2006, 40, 393–397. [Google Scholar] [CrossRef]
- Rafeek, R.; Carrington, C.V.F.; Gomez, A.; Harkins, D.; Torralba, M.; Kuelbs, C. Xylitol and sorbitol effects on the microbiome of saliva and plaque. J. Oral. Microbiol. 2018, 11, 1536181. [Google Scholar] [CrossRef] [Green Version]
- Söderling, E.; Hirvonen, A.; Karjalainen, S.; Fontana, M.; Catt, D.; Seppä, L. The effect of xylitol on the composition of the oral flora: A pilot study. Eur. J. Dent. 2001, 5, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Kayalvizhi, G.; Nivedha, D.; Sajeev, R.; Prathima, G.S.; Suganya, M.; Ramesh, V. Evaluating the Efficacy of Xylitol Wipes on Cariogenic Bacteria in 19- to 35-month-old Children: A Double-blind Randomized Controlled Trial. Int. J. Clin. Pediatr. Dent. 2018, 11, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jeong, S.Y.; Nam, Y.J.; Yang, K.H.; Lim, H.S.; Chung, J. Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis. Clin. Diagn. Lab. Immunol. 2005, 12, 1285–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Park, M.H.; Song, Y.R.; Na, H.S.; Chung, J. Aggregatibacter actinomycetemcomitans-Induced AIM2 Inflammasome Activation Is Suppressed by Xylitol in Differentiated THP-1 Macrophages. J. Periodontol. 2016, 87, e116–e126. [Google Scholar] [CrossRef] [PubMed]
- Beutler, H.O. Xylitol. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers Ltd.: Cambridge, UK, 1988; pp. 484–490. [Google Scholar]
- Loesche, W.J.; Hockett, R.N.; Syed, S.A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch. Oral. Biol. 1972, 17, 1311–1325. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Yaskell, T.; Klepac-Ceraj, V.; Lynch, M.C.; Soukos, N.S. Antimicrobial action of minocycline microspheres versus 810-nm diode laser on human dental plaque microcosm biofilms. J. Periodontol. 2014, 85, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Oliveira, M.; Pasaporti, C.; Heussen, N.; Eduardo, C.P.; Lampert, F.; Apel, C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 2011, 39, 414–421. [Google Scholar] [CrossRef]
- Haffajee, J.A.; Uzel, N.G.; Goodson, J.M. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral. Microbiol. Immunol. 2004, 19, 352–362. [Google Scholar]
- Slots, J.; Slots, H. Bacterial and viral pathogens in saliva: disease relationship and infectious risk. Periodontology 2000 2011, 55, 48–69. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Martin Paster, B.J.; Dewhirst, F.E.; Levin, A.E. “Checkerboard” DNA-DNA hybridization. Biotechniques 1994, 17, 788–792. [Google Scholar]
- Alvim, D.C.S.S.; Ferreira, A.F.M.; Leal, M.A.; Oliveira, L.M.A.; Botelho, A.M.N.; Botelho, A.C.N.; Figueiredo, A.M.S.; Fracalanzza, S.E.L.; Teixeira, L.M.; Pinto, T.C.A. Biofilm production and distribution of pilus variants among Streptococcus agalactiae isolated from human and animal sources. Biofouling 2019, 35, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Contardo, M.S.; Díaz, N.; Lobos, O.; Padilla, C.; Giacaman, R.A. Oral colonization by Streptococcus mutans and its association with the severity of periodontal disease in adults. Rev. Clin. Period. Implantol. Rehabil. Oral. 2011, 4, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Mannaa, A.; Carlén, A.; Campus, G.; Lingström, P. Supragingival plaque microbial analysis in reflection to caries experience. BMC Oral Health 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannaa, A.; Carlén, A.; Dahlén, G.; Lingström, P. Intra-familial comparison of supragingival dental plaque microflora using the checkerboard DNA-DNA hybridisation technique. Arch. Oral. Biol. 2012, 57, 1644–1650. [Google Scholar] [CrossRef]
- Gajendran, J.; Kraemer, J.; Langguth, P. In vivo predictive release methods for medicated chewing gums. Biopharm. Drug Dispos. 2012, 33, 417–424. [Google Scholar] [CrossRef]
- Sjögren, K.; Lingström, P.; Lundberg, A.B.; Birkhed, D. Salivary fluoride concentration and plaque pH after using a fluoride-containing chewing gum. Caries Res. 1997, 31, 366–372. [Google Scholar] [CrossRef]
- Bijella, M.F.; Brighenti, F.L.; Buzalaf, M.A. Fluoride kinetics in saliva after the use of a fluoride-containing chewing gum. Braz. Oral Res. 2005, 19, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Dawes, C.; Macpherson, L.M. Effects of nine different chewing-gums and lozenges on salivary flow rate and pH. Caries Res. 1992, 26, 176–182. [Google Scholar] [CrossRef]
- Alanzi, A.; Soderling, E.; Varghese, A.; Honkala, E. Xylitol Chewing Gums on the Market: Do They Prevent Caries? Oral Health Prev. Dent. 2016, 14, 459–466. [Google Scholar]
- Badet, C.; Furiga, A.; Thébaud, N. Effect of xylitol on an in vitro model of oral biofilm. Oral Health Prev. Dent. 2008, 6, 337–341. [Google Scholar]
- Marya, C.M.; Taneja, P.; Nagpal, R.; Marya, V.; Oberoi, S.S.; Arora, D. Efficacy of Chlorhexidine, Xylitol, and Chlorhexidine + Xylitol against Dental Plaque, Gingivitis, and Salivary Streptococcus mutans Load: A Randomised Controlled Trial. Oral Health Prev. Dent. 2017, 15, 529–536. [Google Scholar]
- Milgrom, P.; Ly, K.A.; Rothen, M. Xylitol and its vehicles for public health needs. Adv. Dent. Res. 2009, 21, 44–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milgrom, P.; Ly, K.A.; Roberts, M.C.; Rothen, M.; Mueller, G.; Yamaguchi, D.K. Mutans streptococci dose response to xylitol chewing gum. J. Dent. Res. 2006, 85, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trahan, L.; Bareil, M.; Gauthier, L.; Vadeboncoeur, C. Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res. 1985, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S. Consistent evidence to support the use of xylitol- and sorbitol-containing chewing gum to prevent dental caries. Evid. Based Dent. 2009, 10, 10–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Lee, Y.J.; Huh, J.; Park, J.W. Synergistic effect of xylitol and ursolic acid combination on oral biofilms. Restor. Dent. Endod. 2004, 39, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyama, M.; Honkala, E.; Honkala, S.; Al-Mutawa, S.A. Effect of xylitol candies on plaque and gingival indices in physically disabled school pupils. J. Clin. Dent. 2006, 17, 17–21. [Google Scholar]
- Shinga-Ishiharaa, S.; Nakaia, Y.; Milgrom, P.; Söderling, E.; Tolvanen, M.; Murakami, K. Xylitol Carry-over Effects on Salivary Mutans Streptococci after 13 Months of Chewing Xylitol Gum. Caries Res. 2012, 46, 519–522. [Google Scholar] [CrossRef]
- Park, E.; Na, H.S.; Kim, S.M.; Wallet, S.; Cha, S.; Chung, J. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis. J. Periodontol. 2014, 85, e212–e223. [Google Scholar] [CrossRef] [Green Version]
- Oscarson, P.; Lif Holgerson, P.; Sjöström, I.; Twetman, S.; Stecksén-Blicks, C. Influence of a low xylitol-dose on mutans streptococci colonisation and caries development in preschool children. Eur. Arch. Paediatr. Dent. 2006, 7, 142–147. [Google Scholar] [CrossRef]
- Wåler, S.M.; Assev, S.; Rölla, G. Xylitol 5-P formation by dental plaque after 12 weeks’ exposure to a xylitol/sorbitol containing chewing gum. Scand. J. Dent. Res. 1992, 6, 319–321. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | 100% Xylitol | 22% Xylitol |
|---|---|---|
| g/% | g/% | |
| Xylitol | 0.903/64.5 | 0.308/22 |
| Sorbitol | - | 0.434/31 |
| Maltitol Syrup | - | 0.084/6 |
| Mannitol | - | 0.056/4 |
| Gum base | 0.421/30.1 | 0.392/28 |
| Other components | 0.076/5.4 | 0.126/9 |
| Time | 100% Xylitol (logμg/L) | 22% Xylitol (logμg/L) | p-Value |
|---|---|---|---|
| Mean ± sd | mean±sd | ||
| Before | −0.68 ± −0.66 | −0.66 ± −0.41 | NS |
| 30 s | 1.49 ± 1.41 | 0.04 ± 1.20 | < 0.01 |
| 1 min | 1.39 ± 1.53 | 1.19 ± 1.38 | 0.02 |
| 2 min | 1.09 ± 1.27 | 1.21 ± 1.24 | 0.03 |
| 5 min | 1.28 ± 1.10 | 1.00 ± 1.19 | 0.02 |
| 10 min | 1.41 ± 1.11 | 0.45 ± 0.64 | < 0.01 |
| 15 min | 1.19 ± 1.12 | −0.08 ± 0.25 | < 0.01 |
| 20 min | 1.07 ± 1.03 | −0.33 ± 0.01 | 0.02 |
| 25 min | −0.30 ± −0.92 | −0.92 ± −1.15 | < 0.01 |
| p-value | < 0.01 | < 0.01 |
| Bacteria Strain | Pre Mean (SD) | 15 min Mean (SD) | 60 min Mean (SD) | 24 h Mean (SD) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| 100% Xylitol | 22% Xylitol | 100% Xylitol | 22% Xylitol | 100% Xylitol | 22% Xylitol | 100% Xylitol | 22% Xylitol | ||
| S. mutans | 3.49 ± 1.08 | 1.76 ± 0.60 | 3.15 ± 0.65 ^ | 1.32 ± 0,47 | 2.82 ± 0.72 ^ | 1.21 ± 0.43 | 2.96 ± 0.56 ^ | p< 0.01 | p = 0.04 |
| S. sobrinus | 2.00 ± 0.65 | 1.20 ± 0.41 | 1.60 ± 0.70 * | 1.11 ± 0.32 | 1.47 ± 0.66 | 1.06 ± 0.24 | 1.74 ± 0.66 ° | p = 0.04 | p = 0.02 |
| P. intermedia | 2.69 ± 1.12 | 1.68 ± 0.64 | 2.34 ± 0.82 ° | 1.53 ± 0.61 | 1.95 ± 0.81 * | 1.29 ± 0.46 | 2.29 ± 0.71 ^ | p < 0.01 | p = 0.05 |
| S. salivarius | 2.11 ± 0.92 | 1.56 ± 0.50 | 1.84 ± 0.69 | 1.32 ± 0.47 | 1.69 ± 0.61 | 1.20 ± 0.41 | 1.91 ± 0.56 ° | p = 0.02 | p = 0.07 |
| S. mitis | 2.46 ± 1.11 | 1.56 ± 0.66 | 1.90 ± 0.75 * | 1.41 ± 0.56 | 1.78 ± 0.65 | 1.12 ± 0.33 | 2.12 ± 0.59 ^ | p < 0.01 | p = 0.02 |
| B. forsytus | 2.32 ± 0.94 | 1.41 ± 0.56 | 2.07 ± 0.51 * | 1.35 ± 0.49 | 1.94 ± 0.50 * | 1.17 ± 0.39 | 1.92 ± 0.47 ° | p = 0.01 | p = 0.09 |
| L. casei | 2.24 ± 1.05 | 1.38 ± 0.55 | 1.76 ± 0.70° | 1.32 ± 0.47 | 1.75 ± 0.60 * | 1.18 ± 0.39 | 2.67 ± 0.51 ^ | p = 0.02 | p = 0.10 |
| L. salivarius | 2.5 ± 1,05 | 1.50 ± 0.62 | 1.95 ± 0.65 * | 1.35 ± 0.48 | 1.68 ± 0.72 | 1.15 ± 0.36 | 2.03 ± 0.66^ | p = 0.01 | p = 0.08 |
| L. fermentum | 2.26 ± 0.90 | 1.38 ± 0.55 | 2.11 ± 0.50 * | 1.26 ± 0.45 | 1.75 ± 0.49 * | 1.21 ± 0.41 | 2.19 ± 0.46 * | p = 0.02 | p = 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, F.; Cagetti, M.G.; Majdub, O.; Campus, G. Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study. Appl. Sci. 2020, 10, 2900. https://doi.org/10.3390/app10082900
Cocco F, Cagetti MG, Majdub O, Campus G. Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study. Applied Sciences. 2020; 10(8):2900. https://doi.org/10.3390/app10082900
Chicago/Turabian StyleCocco, Fabio, Maria Grazia Cagetti, Osama Majdub, and Guglielmo Campus. 2020. "Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study" Applied Sciences 10, no. 8: 2900. https://doi.org/10.3390/app10082900