Growing Biofuel Feedstocks in Copper-Contaminated Soils of a Former Superfund Site
Abstract
:1. Introduction
- To evaluate the effect of compost and plant cover on the fate and geochemical partitioning of Cu.
- To measure the effect of compost and plant cover on erosion of stamp sand.
- To assess the quality of the oil extracted from the seeds of camelina grown on contaminated stamp sand.
2. Materials and Methods
2.1. Soil Sampling, Preparation, and Characterization
2.2. Study Design and Soil Amendments—Phase I Column Studies
2.3. Study Design and Soil Amendments—Phase II Panel Study
2.4. Organic Matter, Carbon, and Nitrogen Analysis
2.5. Oil Content and Quality Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Properties of Stamp Sand and Compost
3.2. Phase I: Greenhouse Column Study
3.2.1. Cu Distribution in Plants (Camelina and Field Pennycress)
3.2.2. Cu Distribution in Leachates and Soil
3.2.3. Plant Growth in the Greenhouse Column Study
3.2.4. Mass Balance of Cu in Greenhouse Column Study
3.3. Phase II: Greenhouse Panel Study
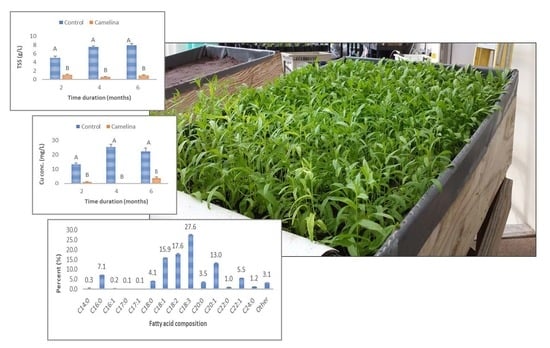
3.3.1. Total Suspended Solids (TSS) and Turbidity in Surface Runoff
3.3.2. Cu in Surface Runoff and Leachate
3.3.3. Cu in Camelina Plants
3.3.4. Cu in Stamp Sands
3.3.5. Mass Balance of Cu in Panel Study
3.3.6. Oil Content and Quality of Camelina Seeds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cusack, C.C.; Mihelcic, J.R. Sediment Toxicity from Copper in the Torch Lake (MI) Great Lakes Area of Concern. J. Great Lakes Res. 1999, 25, 735–743. [Google Scholar] [CrossRef]
- Weston Solutions, Inc. Summary Report for the Torch Lake Area Assessment: Torch Lake NPL Site and Surrounding Areas Keweenaw Peninsula, Michigan; Prepared for US EPA; Document No.: 274-2A-ABDT; Weston Solutions, Inc.: Weston, PA, USA, 2007. [Google Scholar]
- Michigan Department of Community Health (MDCH). Issued a Fish Consumption Advisory on Sauger and Walleye in for Torch Lake, Houghton County based on Fish Tumors of unknown Origin; MDCH: Lansing, MI, USA, 1983.
- Michigan Department of Natural Resources (MDNR). MDNR Remediation Plan for Torch Lake AOC. Surface Water Quality Division, GLEAS; MDNR: Lansing, MI, USA, 1987.
- United States Environmental Protection Agency (USEPA). Torch Lake, Remedial Investigation/Feasibility Study, Houghton County, Michigan, Final Work Plan; US EPA Contract 68-W8-0093; Donohue & Associates, Inc.: Chicago, IL, USA, 1989.
- United States Environmental Protection Agency (USEPA). Comprehensive Environmental Response, Compensation, and Liability Act National Priorities List; USEPA: Washington, DC, USA, 1986.
- Michigan Department of Environmental Quality (MDEQ). Biennial Remedial Action Plan Update for the Torch Lake Area of Concern, Lansing, MI; MDEQ: Lansing, MI, USA, 2007.
- Sidhu, V.; Sarkar, D.; Datta, R. Effects of biosolids and compost amendment on chemistry of soils contaminated with copper from mining activities. Environ. Monit. Assess. 2016, 188, 176. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Methods of Soil Analysis: Part 3. Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2. Comm. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Test Methods for Evaluating Solid Waste. SW 846, Office of Solid Waste and Emergency Response, 3rd ed.; USEPA: Washington, DC, USA, 1996.
- Gee, G.W.; Bauder, J.W. Physical and Mineralogical Methods. Agronomy Monograph No. 9. In Methods of Soil Analysis, Part 1. Particle-size Analysis; Klute, A., Ed.; American Society of Agronomy/Soil Science Society America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight-loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; SSSA Spec. Publ.: Madison, WI, USA, 1996. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2, Agronomy; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Wiley: Hoboken, NJ, USA, 1982; pp. 539–579. [Google Scholar]
- SAS Institute Inc. JMP® Version 10; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Mylavarapu, R.S.; Zinati, G.M. Improvement of soil properties using compost for optimum parsley production in sandy soils. Sci. Hortic. 2009, 120, 426–430. [Google Scholar] [CrossRef]
- Kim, H.-S.; Oh, J.-M.; Luan, S.; Carlson, J.E.; Ahn, S.-J. Cold stress causes rapid but differential changes in properties of plasma membrane H+-ATPase of camelina and rapeseed. J. Plant Physiol. 2013, 170, 828–837. [Google Scholar] [CrossRef]
- Eberle, C.A.; Thom, M.D.; Nemec, K.; Forcella, F.; Lundgren, J.G.; Gesch, R.W.; Riedell, W.E.; Papiernik, S.K.; Wagner, A.; Peterson, D.H.; et al. Using pennycress, camelina, and canola cash cover crops to provision pollinators. Ind. Crop. Prod. 2015, 75, 20–25. [Google Scholar] [CrossRef]
- Groeneveld, J.H.; Klein, A. Pennycress-corn double-cropping increases ground beetle diversity. Biomass Bioenergy 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, Á.; Ördög, A.; Szepesi, Á.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Tordoff, G.M.; Baker, A.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Walker, D.J.; Clemente, R.; Roig, A.; Bernal, M.P. The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ. Pollut. 2003, 122, 303–312. [Google Scholar] [CrossRef]
- Venegas, A.; Rigol, A.; Vidal, M. Effect of ageing on the availability of heavy metals in soils amended with compost and biochar: Evaluation of changes in soil and amendment properties. Environ. Sci. Pollut. Res. 2016, 23, 20619–20627. [Google Scholar] [CrossRef] [Green Version]
- Khokhotva, O.; Waara, S. The influence of dissolved organic carbon on sorption of heavy metals on urea-treated pine bark. J. Hazard. Mater. 2010, 173, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Yingming, L.; Corey, R. Redistribution of Sludge-Borne Cadmium, Copper, and Zinc in a Cultivated Plot. J. Environ. Qual. 1993, 22, 1–8. [Google Scholar] [CrossRef]
- Flores Velez, L.M.; Ducaroir, J.; Jaunet, A.M.; Robert, M. Study of the distribution of copper in an acid sandy vineyard soil by three different methods. Eur. J. Soil Sci. 1996, 47, 523–532. [Google Scholar] [CrossRef]
- Schulin, R.; Geiger, G.; Furrer, G. Heavy metal retention by soil organic matter under changing environmental conditions. In Biogeodynamics of Pollutants in Soils and Sediments: Risk Assessment of Delayed and Nonlinear Responses; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin, Germany, 1995; pp. 53–85. [Google Scholar]
- Hinsinger, P. How do plant roots acquire mineral nutrients chemical processes involved in the rhizosphere? Adv. Agron. 1998, 64, 225–265. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- McLaughlin, M.J.; Smolders, E.; Merckx, R. Soil-root interface physicochemical processes. In Chemistry and Ecosystem Health; Special Publication No. 52; Soil Science Society America: Madison, WI, USA, 1998; pp. 233–277. [Google Scholar]
- Mench, M.; Martin, E. Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 1991, 132, 187–196. [Google Scholar] [CrossRef]
- Mench, M.; Morel, J.L.; Guckert, A. Action des meÂtaux [Cd(II), Cu(II), Pb(II), Zn(II)] sur la production d’exsudats racinaires solubles chez le maõÈs (Zea mays L.). Agronomy 1988, 8, 237–241. [Google Scholar] [CrossRef]
- Treeby, M.; Marschner, H.; RoÈ mheld, V. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 1989, 114, 217–226. [Google Scholar] [CrossRef]
- Cathala, N.; Salsac, L. Absorption du cuivre par les racines demaõÈs (Zea mays L.) et de tournesol (Helianthus annuus L.). Plant Soil 1975, 42, 65–83. [Google Scholar] [CrossRef]
- Songa, J.; Zhaob, F.; Luoa, Y.; McGrathb, S.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Poll. 2004, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M. Introduction to Soil Microbiology, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1977. [Google Scholar]
- Maywald, F.; Weigel, H.J. Biochemistry and molecular biology of heavy metal accumulation in higher plants. Landbauforsch. Volkerode 1997, 47, 103–126. [Google Scholar]
- Moser, B.R.; Vaughn, S.F. Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra-low-sulfur diesel fuel. Biores. Technol. 2010, 101, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Budin, J.T.; Breene, W.M.; Putnam, D.H. Some compositional properties of Camelina (Camelina sativa L. Crantz) seeds and oils. J. Am. Oil Chem. Soc. 1995, 72, 309–315. [Google Scholar] [CrossRef]
- Leonard, E.C. Camelina oil: A-linolenic source. Inform 1998, 9, 830–838. [Google Scholar]
- Sawyer, K. Is there room for camelina? Biodiesel Mag. 2008, 5, 83–87. [Google Scholar]
- Oil World Annual. 2009. Available online: http://www.oilworld.biz/annual (accessed on 5 January 2013).
- Peiretti, P.G.; Meineri, G. Fatty acids, chemical composition and organic matter digestibility of seeds and vegetative parts of false flax (Camelina sativa L.) after different lengths of growth. Anim. Feed Sci. Technol. 2007, 133, 341–350. [Google Scholar] [CrossRef]
- Marquard, V.R.; Kuhlmann, H. Untersuchungen ¨uber ertragslleistung und samenqualit¨at von leindotter (Camelina sativa Crtz.). Fette Seifen Anstrichm. 1986, 88, 245–249. [Google Scholar] [CrossRef]
- Kagale, S.; Koh, C.; Nixon, J.; Bollina, V.; Clarke, W.E.; Tuteja, R.; Spillane, C.; Robinson, S.J.; Links, M.G.; Clarke, C.; et al. The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploidy genome structure. Nat. Commun. 2014, 5, 3706. [Google Scholar] [CrossRef]
- Abramovic, H.; Abram, V. Physico–chemical properties, composition and oxidative stability of Camelina sativa oil. Food Technol. Biotechnol. 2005, 43, 63–70. [Google Scholar]
- Zubr, J. Qualitative variation of Camelina sativa seed from different locations. Ind. Crop. Prod. 2003, 17, 161–169. [Google Scholar] [CrossRef]
- Murphy, E.J. Camelina (Camelina sativa). In Industrial Oil Crops; Mckeon, T.A., Hayes, T.A., Weselake, R.J., Eds.; AOCS press: London, UK, 2016; pp. 207–230. [Google Scholar]
- Huber, G.W.; Iborra, S.; Huber, W. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, S.R.; Prasad, M. Membrane lipid alterations in heavy metal exposed plants. In Heavy Metal Stress in Plants; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Heidelberg, Germany, 1999; pp. 99–116. [Google Scholar]
- Jemal, F.; Zarrouk, M.; Ghorbal, M.H. Effect of cadmium on lipid composition of pepper. Biochem. Soc. Trans. 2000, 28, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Bidar, G.; Verdin, A.; Garcon, G.; Pruvot, C.; Laruelle, F.; Grandmougin- Ferjani, A.; Douay, F.; Shirali, P. Changes in fatty acid composition and content of two plants (Lolium perenne and Trifolium repens) grown during 6 and 18 months in a metal (Pb, Cd, Zn) contaminated field. Water Air Soil Pollut. 2008, 192, 281–291. [Google Scholar] [CrossRef]
- Park, W.; Feng, Y.; Kim, H.; Suh, M.C.; Ahn, S.J. Changes in fatty acid content and composition between wild type and CsHMA3 overexpressing Camelina sativa under heavy-metal stress. Plant Cell Rep. 2015, 34, 1489–1498. [Google Scholar] [CrossRef]
- Beniwal, V.; Aggarwal, H.; Kumar, A.; Chhokar, V. Lipid content and fatty acid change in the developing silique wall of mustard (Brassica juncea L.). Biocatal. Agri. Biotech. 2015, 4, 122–125. [Google Scholar] [CrossRef]
- Rodrıguez-Rodrıguez, M.F.; Sanchez-Garcia, A.; Salas, J.J.; Garces, R.; Martınez-Force, E. Characterization of the morphological changes and fatty acid profile of developing Camelina sativa seeds. Ind. Crop Prod. 2013, 50, 673–679. [Google Scholar] [CrossRef]





| Properties | Stamp Sand | Compost |
|---|---|---|
| pH | 8.2 | 6.9 |
| Electrical Conductivity (μS/cm) | 83 | 1840 |
| Organic matter (%) | 0.5 | 84 |
| Sand (%) | 95 | NA |
| Silt (%) | 2.5 | NA |
| Clay (%) | 2.5 | NA |
| Cu (mg/kg) | 719 | 54 |
| Fe (mg/kg) | 6200 | BDL |
| Al (mg/kg) | 2300 | BDL |
| Plant Available P (mg/kg) | 0.70 | 1009 |
| Total P (mg/kg) | 800 | 2090 |
| Total N (g/kg) | 1.81 | 388 |
| Plant Species | Roots | Stem | Leaves | Pods | Seeds | Total Biomass |
|---|---|---|---|---|---|---|
| Camelina | ||||||
| Compost–2.5% | 0.08c | 0.96c | 0.47c | 0.12d | 0.09c | 1.72e |
| Compost–5% | 0.14c | 0.97c | 0.58c | 0.05d | 0.04bc | 1.78e |
| Compost–10% | 0.12c | 2.10b | 0.85c | 0.51b | 0.37bc | 3.96cd |
| Compost–20% | 0.44bc | 3.53a | 2.86b | 0.18cd | 0.19bc | 7.21b |
| Field Pennycress | ||||||
| Compost–2.5% | 0.11c | 0.50c | 0.68c | 0.24cd | 0.21bc | 1.74e |
| Compost–5% | 0.11c | 0.81c | 1.31c | 0.35bc | 0.30bc | 2.89de |
| Compost–10% | 0.78ab | 0.81c | 2.76b | 0.53b | 0.65b | 5.53c |
| Compost–20% | 1.03ab | 2.26b | 8.05a | 1.40a | 1.30a | 14.0a |
| Roots (mg) | Stem (mg) | Leaves (mg) | Pods (mg) | Seeds (mg) | Total Plant Uptake (mg) | Total Cu (Leachates; mg) | Total Cu (Soil; mg) | |
|---|---|---|---|---|---|---|---|---|
| Control (No plant) | - | - | - | - | - | - | 0.05 | 1757 |
| Camelina | ||||||||
| Compost–2.5% | 0.02 | 0.06 | 0.01 | 0.01 | 0.01 | 0.11 | 8.69 | 1733 |
| Compost–5% | 0.04 | 0.06 | 0.12 | 0.01 | 0.003 | 0.23 | 20.0 | 1709 |
| Compost–10% | 0.02 | 0.08 | 0.14 | 0.02 | 0.02 | 0.28 | 43.3 | 1671 |
| Compost–20% | 0.07 | 0.17 | 0.34 | 0.01 | 0.01 | 0.60 | 131 | 1550 |
| Field Pennycress | ||||||||
| Compost–2.5% | 0.02 | 0.01 | 0.07 | 0.04 | 0.01 | 0.15 | 5.78 | 1719 |
| Compost–5% | 0.03 | 0.02 | 0.19 | 0.04 | 0.01 | 0.29 | 14.4 | 1697 |
| Compost–10% | 0.08 | 0.02 | 0.16 | 0.05 | 0.02 | 0.33 | 37.4 | 1654 |
| Compost–20% | 0.15 | 0.03 | 0.37 | 0.04 | 0.03 | 0.62 | 106 | 1570 |
| Roots | Stem | Leaves | Pods | Seeds | Total plant uptake | Leachates | Surface Runoff | Soil | |
|---|---|---|---|---|---|---|---|---|---|
| Control (No plant) | - | - | - | - | - | - | 90 | 1786 | 76,565 |
| Camelina | 2.48 | 1.90 | 3.84 | 0.34 | 0.63 | 9.2 | 84 | 123 | 78,545 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, V.; Sarkar, D.; Datta, R. Growing Biofuel Feedstocks in Copper-Contaminated Soils of a Former Superfund Site. Appl. Sci. 2020, 10, 1499. https://doi.org/10.3390/app10041499
Sidhu V, Sarkar D, Datta R. Growing Biofuel Feedstocks in Copper-Contaminated Soils of a Former Superfund Site. Applied Sciences. 2020; 10(4):1499. https://doi.org/10.3390/app10041499
Chicago/Turabian StyleSidhu, Virinder, Dibyendu Sarkar, and Rupali Datta. 2020. "Growing Biofuel Feedstocks in Copper-Contaminated Soils of a Former Superfund Site" Applied Sciences 10, no. 4: 1499. https://doi.org/10.3390/app10041499