Imaging System Based on Silicon Photomultipliers and Light Emitting Diodes for Functional Near-Infrared Spectroscopy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. System Architecture
2.2. Phantom Optical Characterization
2.3. System Functioning and Components
3. Results and Discussion
3.1. Data Correction
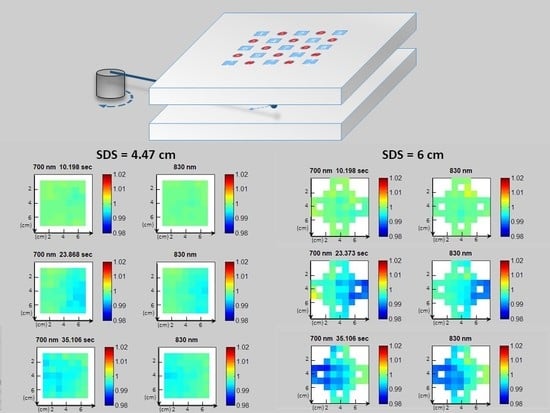
- Differences on the value of the photocurrents related to channels with the same SDS, as shown in Figure 8a. They are due to little displacements of the LEDs or of the SiPMs, small differences in the EQE of the different SiPMs involved, or differences among LEDs’ brightness.
- Drifts over time of the photocurrents, as shown in Figure 8b. To explain the decrease over time of the photocurrents recorded on the static phantom, it is important to consider that we subtract the dark current to determine the SiPM signal. In general, at a constant bias voltage, as SiPM temperature increases, both the photocurrent and the dark current in a SiPM increase while the gain slightly decreases. Moreover, the light output of an LED at a constant current also decreases with the increase of its junction temperature [30]. All this instability effects the sum up giving rise to the overall slow drift effects shown in Figure 8.
3.2. Image Reconstruction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrig, H.; Villringer, A. Beyond the visible-imaging the human brain with light. J. Cerebr. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Rui, G.; Chen, W.; Li, S.; Schulz, P.E.; Zhang, Y. Early Detection of Alzheimer’s Disease Using Non-invasive Near-Infrared Spectroscopy. Front. Aging Neurosci. 2018, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, H.; Yu, D. Novel analysis of fNIRS acquired dynamic hemoglobin concentrations: Application in young children with autism spectrum disorder. Biomed. Opt. Express. 2018, 9, 3694–3710. [Google Scholar] [CrossRef]
- Aries, M.J.; Coumou, A.D.; Elting, J.W.; van der Harst, J.J.; Kremer, B.P.; Vroomen, P.C. Near Infrared Spectroscopy for the Detection of Desaturations in Vulnerable Ischemic Brain Tissue: A Pilot Study at the Stroke Unit Bedside. Stroke 2012, 43, 1134–1136. [Google Scholar] [CrossRef]
- Stojanovic-Radic, J.; Wylie, G.; Voelbel, G.; Chiaravalloti, N.; DeLuca, J. Neuroimaging and cognition using functional near infrared spectroscopy (fNIRS) in multiple sclerosis. Brain Imaging Behav. 2015, 9, 302–311. [Google Scholar] [CrossRef]
- Wolf, M.; Ferrari, M.; Quaresima, V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 2007, 12, 062104. [Google Scholar] [CrossRef]
- Strangman, G.E.; Li, Z.; Zhang, Q. Depth sensitivity and source-detector separations for near infrared spectroscopy based on the Colin27 brain template. PLoS ONE 2013, 8, e66319. [Google Scholar] [CrossRef] [Green Version]
- White, B.R.; Culver, J.P. Quantitative evaluation of high-density diffuse optical tomography: In vivo resolution and mapping performance. J. Biomed. Opt. 2010, 15, 026006. [Google Scholar] [CrossRef]
- Zeff, B.W.; White, B.R.; Dehghani, H.; Schlaggar, B.L.; Culver, J.P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc. Natl. Acad. Sci. USA 2007, 104, 12169–12174. [Google Scholar] [CrossRef] [Green Version]
- Joseph, D.K.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Diffuse optical tomography system to image brain activation with improved spatial resolution and validation with functional magnetic resonance imaging. Appl. Opt. 2006, 45, 8142–8151. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.P.; Habermehl, C.; Mehnert, J.; Schmitz, C.H.; Holtze, S.; Villringer, A.; Steinbrink, J.; Obrig, H. High-resolution optical functional mapping of the human somatosensory cortex. Front. Neuroenerg. 2010, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggebrecht, A.T.; Ferradal, S.L.; Robichaux-Viehoever, A.; Hassanpour, M.S.; Dehghani, H.; Snyder, A.Z.; Hershey, T.; Culver, J.P. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photonics 2014, 8, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sieno, L.; Bettega, G.; Berger, M.; Hamou, C.; Aribert, M.; Dalla Mora, A.; Puszka, A.; Grateau, H.; Contini, D.; Hervé, L.; et al. Toward non-invasive assessment of flap viability with time-resolved diffuse optical tomography: A preclinical test on rats. J. Biomed. Opt. 2016, 21, 25004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, A.M.; Libertino, S.; Zappasodi, F.; Mazzillo, M.; Di Pompeo, F.; Merla, A.; Lombardo, S.; Fallica, G. Characterization of a fiber-less, multichannel optical probe for continuous wave functional near-infrared spectroscopy based on silicon photomultipliers detectors: In-vivo assessment of primary sensorimotor response. Neurophotonics 2017, 4, 035002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, R.; Libertino, S.; Sanfilippo, D.; Fallica, G.; Lombardo, S. Improvement of sensitivity in continuous wave near infrared spectroscopy systems by using silicon photomultipliers. Biomed. Opt. Express 2016, 7, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyser, D.; Lambercy, O.; Scholkmann, F.; Wolf, M.; Gassert, R. Wearable and modular functional nearinfrared spectroscopy instrument with multidistance measurements at four wavelengths. Neurophotonics 2017, 4, 041413. [Google Scholar] [CrossRef]
- Zimmermann, R.; Braun, F.; Achtnich, T.; Lambercy, O.; Gassert, R.; Wolf, M. Silicon photomultipliers for improved detection of low light levels in miniature near-infrared spectroscopy instruments. Biomed. Opt. Express 2013, 4, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Maira, G.; Mazzillo, M.; Libertino, S.; Fallica, G.; Lombardo, S. Crucial aspects for the use of silicon photomultiplier devices in continuous wave functional near-infrared spectroscopy. Biomed. Opt Express 2018, 9, 4679–4688. [Google Scholar] [CrossRef]
- Farina, A.; Tagliabue, S.; Di Sieno, L.; Martinenghi, E.; Durduran, T.; Arridge, S.; Martelli, F.; Torricelli, A.; Pifferi, A.; Dalla Mora, A. Time-Domain Functional Diffuse Optical Tomography System Based on Fiber-Free Silicon Photomultipliers. Appl. Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Martinenghi, E.; Dalla Mora, A.; Contini, D.; Pifferi, A.; Torricelli, A. Probe-hosted silicon photomultipliers for time-domain functional near-infrared spectroscopy: Phantom and in vivo tests. Neurophotonics 2016, 3, 045004. [Google Scholar] [CrossRef] [Green Version]
- Boas, D.A.; Chen, K.; Grebert, D.; Franceschini, M.A. Improving the diffuse optical imaging spatial resolution of the cerebral hemodynamic response to brain activation in humans. Opt. Lett. 2004, 29, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Pagano, R.; Corso, D.; Lombardo, S.; Valvo, G.; Sanfilippo, D.; Fallica, G.; Libertino, S. Dark Current in Silicon Photomultiplier Pixels: Data and Model. IEEE Trans. Electron. Devices 2012, 59, 2410. [Google Scholar] [CrossRef]
- Zijlstra, W.G.; Buursma, A.; Van Assendelft, O.W. Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application; CRC Press: Zeist, The Netherlands, 2000. [Google Scholar]
- Farina, A.; Torricelli, A.; Bargigia, I.; Spinelli, L.; Cubeddu, R.; Foschum, F.; Jager, M.; Simon, E.; Fugger, O.; Kienle, A.; et al. In-vivo multilaboratory investigation of the optical properties of the human head. Biomed. Opt. Express 2015, 6, 2609–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholkmann, F.; Wolf, M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Fantini, S.; Fabiani, M.; Gratton, G. Low resolution mapping of the effective attenuation coefficient of the human head: A multi-distance approach applied to high-density optical recordings. Neurophotonics 2017, 4, 021103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arridge, S.R.; Cope, M.; Delpy, D.T. The theoretical basis for the determination of optical pathlengths in tissue: Temporal and frequency analysis. Phys. Med. Biol. 1992, 37, 1531. [Google Scholar] [CrossRef]
- On Semiconductor MICROFJ-60035 Datasheet. Available online: www.onsemi.com/pub/Collateral/MICROJ-SERIES-D.PDF (accessed on 10 January 2020).
- Sá, E.M.; Antunes, F.L.M.; Perin, A.J. Junction Temperature Estimation for High Power Light-Emitting Diodes. In Proceedings of the IEEE International Symposium on Industrial Electronics, Centro Cultural and Social Caixanova, Vigo, Spain, 4–7 June 2007. [Google Scholar] [CrossRef]
- Arridge, S.R. Optical Tomography in medical imaging. Inverse Probl. 1999, 15, R41–R93. [Google Scholar] [CrossRef] [Green Version]
- Arridge, S.R. Methods in diffuse optical imaging. Philos. Trans. R. Soc. A 2011, 369, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Arridge, S.R.; Schweiger, M. Photon-measurement density functions. Part 2: Finite-element-method calculations. Appl. Opt. 1995, 34, 8026–8037. [Google Scholar] [CrossRef] [Green Version]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse Optics for Tissue Monitoring and Tomography. Rep. Prog. Phys. 2010, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Mathewson, K.E.; Fabiani, M.; Gratton, G. Combining energy and Laplacian regularization to accurately retrieve the depth of brain activity of diffuse optical tomographic data. J. Biomed. Opt. 2016, 21, 036008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschini, M.A.; Toronov, V.; Filiaci, M.E.; Gratton, E.; Fantini, S. On-line optical imaging of the human brain with 160-ms temporal resolution. Opt. Express 2000, 6, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maira, G.; Chiarelli, A.M.; Brafa, S.; Libertino, S.; Fallica, G.; Merla, A.; Lombardo, S. Imaging System Based on Silicon Photomultipliers and Light Emitting Diodes for Functional Near-Infrared Spectroscopy. Appl. Sci. 2020, 10, 1068. https://doi.org/10.3390/app10031068
Maira G, Chiarelli AM, Brafa S, Libertino S, Fallica G, Merla A, Lombardo S. Imaging System Based on Silicon Photomultipliers and Light Emitting Diodes for Functional Near-Infrared Spectroscopy. Applied Sciences. 2020; 10(3):1068. https://doi.org/10.3390/app10031068
Chicago/Turabian StyleMaira, Giovanni, Antonio M. Chiarelli, Stefano Brafa, Sebania Libertino, Giorgio Fallica, Arcangelo Merla, and Salvatore Lombardo. 2020. "Imaging System Based on Silicon Photomultipliers and Light Emitting Diodes for Functional Near-Infrared Spectroscopy" Applied Sciences 10, no. 3: 1068. https://doi.org/10.3390/app10031068