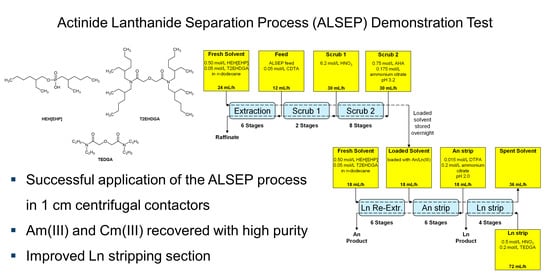
Countercurrent Actinide Lanthanide Separation Process (ALSEP) Demonstration Test with a Simulated PUREX Raffinate in Centrifugal Contactors on the Laboratory Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Centrifugal Contactor Setup
2.3. Procedures and Analytics
3. Results and Discussion
3.1. Single-Centrifugal Contactor Tests and Radiochemical Analyses
3.1.1. Extraction Section
3.1.2. Scrub 2 Section
3.1.3. An Stripping Section
3.1.4. Ln Re-Extraction Section
3.1.5. Ln Stripping Batch Tests
3.1.6. Ln Stripping Section
3.2. Flowsheet Calculations Using the AMUSE Code
- The co-extraction section was reduced to 6 stages and scrub 2 section increased to 8 stages to enable more complete Mo scrubbing.
- Distribution data from single-stage contactor tests were used in the AMUSE code with no further adjustment of stage efficiency.
- Results using the final D values (steady state) as well as with average D values (excluding first and second samples) during the single-stage tests were both used to evaluate recovery.
- Scrub 1 D values were assumed the same as the extraction D values.
3.3. Full Countercurrent Test and Radiochemical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, R.J. (Ed.) Reprocessing and Recycling of Spent Nuclear Fuel; Woodhead Publishing: Cambridge, UK, C2013-0-16483-5; 2015. [Google Scholar]
- OECD-NEA. Transition towards a Sustainable Nuclear Fuel Cycle; NEA No. 7133; OECD Nuclear Energy Agency: Paris, France, 2013. [Google Scholar]
- OECD-NEA. State-of-the-Art Report on the Progress of Nuclear Fuel Cycle Chemistry; NEA No. 7267; OECD Nuclear Energy Agency: Paris, France, 2018. [Google Scholar]
- Taylor, R.; Bourg, S.; Glatz, J.-P.; Modolo, G. Development of actinide separation processes for future nuclear fuel cycles in Europe. Nucl. Future 2015, 11, 38–43. [Google Scholar]
- Moyer, B.A.; Lumetta, G.J.; Mincher, B.J. Minor actinide separation in the reprocessing of spent nuclear fuels: Recent advances in the United States. In Reprocessing and Recycling of Spent Nuclear Fuel; Taylor, R., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 289–312. [Google Scholar] [CrossRef]
- Poinssot, C.; Bourg, S.; Ouvrier, N.; Combernoux, N.; Rostaing, C.; Vargas-Gonzalez, M.; Bruno, J. Assessment of the environmental footprint of nuclear energy systems. Comparison between closed and open fuel cycles. Energy 2014, 69, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Poinssot, C.; Bourg, S.; Boullis, B. Improving the nuclear energy sustainability by decreasing its environmental footprint. Guidelines from life cycle assessment simulations. Prog. Nucl. Energy 2016, 92, 234–241. [Google Scholar] [CrossRef]
- Serp, J.; Poinssot, C.; Bourg, S. Assessment of the Anticipated Environmental Footprint of Future Nuclear Energy Systems. Evidence of the Beneficial Effect of Extensive Recycling. Energies 2017, 10, 1445. [Google Scholar] [CrossRef] [Green Version]
- Lanham, W.B.; Runion, T.C. PUREX Process for Plutonium and Uranium Recovery; ORNL-479; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1949. [Google Scholar]
- Dinh, B.; Moisy, P.; Baron, P.; Calor, J.-N.; Espinoux, D.; Lorrain, B.; Benchikouhne-Ranchoux, M. Modified PUREX first-cycle extraction for neptunium recovery. In Proceedings of the 18th International Solvent Extraction Conference (ISEC), Tuscon, AZ, USA, 15–19 September 2008; pp. 581–586. [Google Scholar]
- Taylor, R.J.; Gregson, C.R.; Carrott, M.J.; Mason, C.; Sarsfield, M.J. Progress towards the Full Recovery of Neptunium in an Advanced PUREX Process. Solvent Extr. Ion Exch. 2013, 31, 442–462. [Google Scholar] [CrossRef]
- Modolo, G.; Wilden, A.; Geist, A.; Magnusson, D.; Malmbeck, R. A review of the demonstration of innovative solvent extraction processes for the recovery of trivalent minor actinides from PUREX raffinate. Radiochim. Acta 2012, 100, 715–725. [Google Scholar] [CrossRef]
- Modolo, G.; Geist, A.; Miguirditchian, M. Minor actinide separations in the reprocessing of spent nuclear fuels: Recent advances in Europe. In Reprocessing and Recycling of Spent Nuclear Fuel; Taylor, R., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 245–287. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J. Separation of Actinides from Spent Nuclear Fuel: A Review. J. Hazard. Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef]
- Baron, P.; Cornet, S.M.; Collins, E.D.; DeAngelis, G.; Del Cul, G.; Fedorov, Y.; Glatz, J.P.; Ignatiev, V.; Inoue, T.; Khaperskaya, A.; et al. A review of separation processes proposed for advanced fuel cycles based on technology readiness level assessments. Prog. Nucl. Energy 2019, 117, 24. [Google Scholar] [CrossRef]
- Miguirditchian, M.; Vanel, V.; Marie, C.; Pacary, V.; Charbonnel, M.-C.; Berthon, L.; Hérès, X.; Montuir, M.; Sorel, C.; Bollesteros, M.-J.; et al. Americium Recovery from Highly Active PUREX Raffinate by Solvent Extraction: The EXAm Process. A Review of 10 Years of R&D. Solvent Extr. Ion Exch. 2020, 38, 365–387. [Google Scholar] [CrossRef]
- Wilden, A.; Modolo, G.; Schreinemachers, C.; Sadowski, F.; Lange, S.; Sypula, M.; Magnusson, D.; Geist, A.; Lewis, F.W.; Harwood, L.M.; et al. Direct Selective Extraction of Actinides (III) from PUREX Raffinate using a Mixture of CyMe4BTBP and TODGA as 1-cycle SANEX Solvent Part III: Demonstration of a Laboratory-scale Counter-Current Centrifugal Contactor Process. Solvent Extr. Ion Exch. 2013, 31, 519–537. [Google Scholar] [CrossRef]
- Gelis, A.V.; Lumetta, G.J. Actinide Lanthanide Separation Process-ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. [Google Scholar] [CrossRef]
- Wilden, A.; Modolo, G.; Kaufholz, P.; Sadowski, F.; Lange, S.; Munzel, D.; Geist, A. Process Development and Laboratory-scale Demonstration of a regular-SANEX Process Using C5-BPP. Sep. Sci. Technol. 2015, 50, 2467–2475. [Google Scholar] [CrossRef]
- Wilden, A.; Modolo, G.; Kaufholz, P.; Sadowski, F.; Lange, S.; Sypula, M.; Magnusson, D.; Müllich, U.; Geist, A.; Bosbach, D. Laboratory-Scale Counter-Current Centrifugal Contactor Demonstration of an Innovative-SANEX Process Using a Water Soluble BTP. Solvent Extr. Ion Exch. 2015, 33, 91–108. [Google Scholar] [CrossRef]
- Carrott, M.; Maher, C.; Mason, C.; Sarsfield, M.; Taylor, R. “TRU-SANEX”: A variation on the EURO-GANEX and i-SANEX processes for heterogeneous recycling of actinides Np-Cm. Sep. Sci. Technol. 2016, 51, 2198–2213. [Google Scholar] [CrossRef]
- Marie, C.; Kaufholz, P.; Vanel, V.; Duchesne, M.-T.; Russello, E.; Faroldi, F.; Baldini, L.; Casnati, A.; Wilden, A.; Modolo, G.; et al. Development of a Selective Americium Separation Process Using H4TPAEN as Water-Soluble Stripping Agent. Solvent Extr. Ion Exch. 2019, 37, 313–327. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Gelis, A.V.; Carter, J.C.; Niver, C.M.; Smoot, M.R. The Actinide-Lanthanide Separation Concept. Solvent Extr. Ion Exch. 2014, 32, 333–347. [Google Scholar] [CrossRef]
- Brown, M.A.; Wardle, K.E.; Lumetta, G.; Gelis, A.V. Accomplishing Equilibrium in ALSEP: Demonstrations of Modified Process Chemistry on 3-D Printed Enhanced Annular Centrifugal Contactors. Procedia Chem. 2016, 21, 167–173. [Google Scholar] [CrossRef]
- Holfeltz, V.E.; Campbell, E.L.; Peterman, D.R.; Standaert, R.F.; Paulenova, A.; Lumetta, G.J.; Levitskaia, T.G. Effect of HEH[EHP] impurities on the ALSEP solvent extraction process. Solvent Extr. Ion Exch. 2017, 36, 22–40. [Google Scholar] [CrossRef]
- Peterman, D.R.; Zarzana, C.A.; Tillotson, R.D.; McDowell, R.G.; Rae, C.; Groenewold, G.S.; Law, J.D. Evaluation of the impacts of gamma radiolysis on an ALSEP process solvent. J. Radioanal. Nucl. Chem. 2018, 316, 855–860. [Google Scholar] [CrossRef]
- Gelis, A.V.; Kozak, P.; Breshears, A.T.; Brown, M.A.; Launiere, C.; Campbell, E.L.; Hall, G.B.; Levitskaia, T.G.; Holfeltz, V.E.; Lumetta, G.J. Closing the Nuclear Fuel Cycle with a Simplified Minor Actinide Lanthanide Separation Process (ALSEP) and Additive Manufacturing. Sci. Rep. 2019, 9, 12842. [Google Scholar] [CrossRef]
- Picayo, G.A.; Etz, B.D.; Vyas, S.; Jensen, M.P. Characterization of the ALSEP Process at Equilibrium: Speciation and Stoichiometry of the Extracted Complex. ACS Omega 2020, 5, 8076–8089. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.B.; Holfeltz, V.E.; Campbell, E.L.; Boglaienko, D.; Lumetta, G.J.; Levitskaia, T.G. Evolution of Acid-Dependent Am3+ and Eu3+ Organic Coordination Environment: Effects on the Extraction Efficiency. Inorg. Chem. 2020, 59, 4453–4467. [Google Scholar] [CrossRef] [PubMed]
- Sypula, M.; Wilden, A.; Schreinemachers, C.; Malmbeck, R.; Geist, A.; Taylor, R.; Modolo, G. Use of polyaminocarboxylic acids as hydrophilic masking agents for fission products in actinide partitioning processes. Solvent Extr. Ion Exch. 2012, 30, 748–764. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Allred, J.R.; Bryan, S.A.; Hall, G.B.; Levitskaia, T.G.; Lines, A.M.; Sinkov, S.I. Simulant Testing of a Co-decontamination (CoDCon) Flowsheet for a Product with a Controlled Uranium-to-Plutonium Ratio. Sep. Sci. Technol. 2019, 54, 1977–1984. [Google Scholar] [CrossRef]
- Hu, Z.; Pan, Y.; Ma, W.; Fu, X. Purification of Organophosphorus Acid Extractants. Solvent Extr. Ion Exch. 1995, 13, 965–976. [Google Scholar] [CrossRef]
- Leonard, R.A. Design Principles and Applications of Centrifugal Contactors for Solvent Extraction. In Ion Exchange and Solvent Extraction, A Series of Advances; Moyer, B.A., Ed.; CRC Taylor and Francis: Boca Raton, FL, USA, 2009; Volume 19, pp. 563–616. [Google Scholar]
- Duan, W.; Zhao, M.; Wang, C.; Cao, S. Recent Advances in the Development and Application of Annular Centrifugal Contactors in the Nuclear Industry. Solvent Extr. Ion Exch. 2014, 32, 1–26. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Campbell, E.L.; Casella, A.J.; Hall, G.B.; Holfeltz, V.E.; Levitskaia, T.G. Sigma Team for Advanced Actinide Recovery: PNNL FY 2016 Summary Report; FCRD-MRWFD-2016-000324; U.S. Department of Energy, Sigma Team for Advanced Actinide Recovery, Pacific Northwest National Laboratory: Richland, WA, USA, 2016.
- Chapron, S.; Marie, C.; Arrachart, G.; Miguirditchian, M.; Pellet-Rostaing, S. New Insight into the Americium/Curium Separation by Solvent Extraction Using Diglycolamides. Solvent Extr. Ion Exch. 2015, 33, 236–248. [Google Scholar] [CrossRef]
- Herdzik-Koniecko, I.; Wagner, C.; Trumm, M.; Müllich, U.; Schimmelpfennig, B.; Narbutt, J.; Geist, A.; Panak, P.J. Do An(III) and Ln(III) ions form heteroleptic complexes with diglycolamide and hydrophilic BT(B)P ligands in solvent extraction systems? A spectroscopic and DFT study. New J. Chem. 2019, 43, 6314–6322. [Google Scholar] [CrossRef] [Green Version]
- Klaß, L.; Wilden, A.; Kreft, F.; Wagner, C.; Geist, A.; Panak, P.J.; Herdzik-Koniecko, I.; Narbutt, J.; Modolo, G. Evaluation of the Hydrophilic Complexant N,N,N′,N′-tetraethyldiglycolamide (TEDGA) and its Methyl-substituted Analogues in the Selective Am(III) Separation. Solvent Extr. Ion Exch. 2019, 37, 297–312. [Google Scholar] [CrossRef]
- Frey, K.; Krebs, J.F.; Pereira, C. Time-Dependent Implementation of Argonne’s Model for Universal Solvent Extraction. Ind. Eng. Chem. Res. 2012, 51, 13219–13226. [Google Scholar] [CrossRef]










| Element | Concentration ALSEP Feed [mg L−1] * | Element | Concentration ALSEP Feed [mg L−1] * |
|---|---|---|---|
| Fe | 7 | Cs | 665 |
| Rb | 90 | La | 309 |
| Sr | 207 | Ce | 591 |
| Y | 119 | Pr | 267 |
| Zr | 718 | Nd | 1003 |
| Mo | 425 | Sm | 213 |
| Ru | 296 | Eu | 44 |
| Rh | 1 | Gd | 45 |
| Pd | 6 | 241Am | 3.1 MBq L−1 |
| Sn | 14 | 244Cm | 3.0 MBq L−1 |
| Te | 69 | 152Eu | 5.6 MBq L−1 |
| HNO3 | 2.9 mol L−1 |
| Section | Aq. Flow Rate [mL h−1] | Org. Flow Rate [mL h−1] | Contactor Filled with * |
|---|---|---|---|
| Extraction | 72 | 24 | 3 mol L−1 HNO3 |
| Mo scrubbing (Scrub 2) | 30 | 24 | Fresh Scrub 2 solution |
| MA stripping | 18 | 36 | Fresh Strip 1 solution |
| Ln re-extraction | 18 | 18 | Fresh Strip 1 solution |
| Ln stripping | 54 | 36 | Fresh 0.5 mol L−1 TEDGA in 0.5 mol L−1 HNO3 |
| Recovery in the Loaded Solvent after Scrub 2 | ||
|---|---|---|
| Steady State | Average | |
| Am | 99.87% | 99.92% |
| Eu | >99.99% | >99.99% |
| Cm | 99.99% | 99.97% |
| La | 10.00% | 10.70% |
| Ce | 70.20% | 72.80% |
| Pr | 96.90% | 97.40% |
| Nd | 99.70% | 99.80% |
| Sm | >99.99% | >99.99% |
| Eu | >99.99% | >99.99% |
| Gd | >99.99% | >99.98% |
| Mo | <0.01% | <0.01% |
| Fe | 0.64% | 0.16% |
| Zr | <0.01% | 0.01% |
| Ru | <0.01% | <0.01% |
| Rb | <0.01% | <0.01% |
| Sr | <0.01% | <0.01% |
| Cs | <0.01% | <0.01% |
| Y | 47.00% | 78.00% |
| Rh | <0.01% | <0.01% |
| Pd | <0.01% | <0.01% |
| Sn | <0.01% | <0.01% |
| Te | <0.01% | <0.01% |
| Element | Raffinate [%] | Loaded Solvent [%] | An Product [%] | Ln Product [%] | Spent Solvent [%] | DFfeed/An product | DFAm/M | DFCm/M | CFproduct/feed |
|---|---|---|---|---|---|---|---|---|---|
| 241Am | <0.1 | >99.9 | 95.7 | 4.3 | <0.1 | 1.04 | 1.00 | 0.99 | 0.48 |
| 244Cm | <0.1 | >99.9 | 94.3 | 5.7 | <0.1 | 1.06 | 1.01 | 1.00 | 0.47 |
| Fe * | 86.5 | 8.4 | 6.0 | 3.0 | 12.1 | 16.7 | 16.0 | 15.7 | 0.03 |
| Rb | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Sr | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Y | <0.1 | >99.9 | <0.1 | >99.9 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Zr | 99.8 | 0.2 | 0.2 | <0.1 | <0.1 | ≥500 | ≥479 | ≥472 | ≤0.001 |
| Mo | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Ru | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Rh | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Pd | 99.6 | 0.4 | 0.1 | 0.2 | 0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Sn | 99.1 | 0.9 | 0.1 | <0.1 | 0.8 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Te | 98.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Cs | >99.9 | <0.1 | <0.1 | <0.1 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| La | 94.1 | 5.9 | <0.1 | 5.9 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Ce | 16.3 | 83.7 | <0.1 | 83.7 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Pr | 0.6 | 99.4 | <0.1 | 99.4 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Nd | 0.1 | 99.9 | <0.1 | 99.9 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Sm | <0.1 | >99.9 | <0.1 | >99.9 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Eu | <0.1 | >99.9 | <0.1 | >99.9 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
| Gd | 0.6 | 99.4 | <0.1 | 99.4 | <0.1 | ≥1000 | ≥957 | ≥943 | ≤0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilden, A.; Kreft, F.; Schneider, D.; Paparigas, Z.; Modolo, G.; Lumetta, G.J.; Gelis, A.V.; Law, J.D.; Geist, A. Countercurrent Actinide Lanthanide Separation Process (ALSEP) Demonstration Test with a Simulated PUREX Raffinate in Centrifugal Contactors on the Laboratory Scale. Appl. Sci. 2020, 10, 7217. https://doi.org/10.3390/app10207217
Wilden A, Kreft F, Schneider D, Paparigas Z, Modolo G, Lumetta GJ, Gelis AV, Law JD, Geist A. Countercurrent Actinide Lanthanide Separation Process (ALSEP) Demonstration Test with a Simulated PUREX Raffinate in Centrifugal Contactors on the Laboratory Scale. Applied Sciences. 2020; 10(20):7217. https://doi.org/10.3390/app10207217
Chicago/Turabian StyleWilden, Andreas, Fabian Kreft, Dimitri Schneider, Zaina Paparigas, Giuseppe Modolo, Gregg J. Lumetta, Artem V. Gelis, Jack D. Law, and Andreas Geist. 2020. "Countercurrent Actinide Lanthanide Separation Process (ALSEP) Demonstration Test with a Simulated PUREX Raffinate in Centrifugal Contactors on the Laboratory Scale" Applied Sciences 10, no. 20: 7217. https://doi.org/10.3390/app10207217