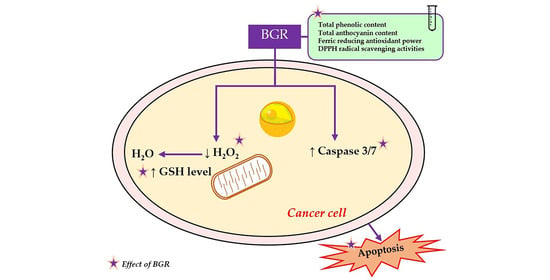
Effect of Harvest Age on Total Phenolic, Total Anthocyanin Content, Bioactive Antioxidant Capacity and Antiproliferation of Black and White Glutinous Rice Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Plant Extraction
2.3. Total Phenolic Content (TPC) by Folin–Ciocalteu’s Reagent Method
2.4. Total Anthocyanin Content (TAC)
- A510 and A700 are the absorbances at 510 nm and 700 nm,
- Adiff = (A510 − A700) pH 1.0 − (A510 − A700) pH 4.5,
- Mw is the molecular weight of cyanidin 3-glucoside (C3GE), 449.2 g/mol,
- DF, a dilution factor (10),
- 1000, the conversion factor 1000 mg/g,
- ε is the molar extinction coefficient for 26,900 L mol−1 cm−1.
2.5. DPPH Radical Scavenging Assay
2.6. Reducing Antioxidant Power Based on FRAP Assay
2.7. Cytotoxicity Assay by Neutral Red Assay
2.8. Determination of Intracellular Peroxide (H2O2) by DCFH-DA Assay
2.9. Determination of the Total Endogenous Glutathione Level
2.10. Apoptotic Cell Death Mode by Flow Cytometry
2.11. Caspase 3/7 Activity Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield of Rice Sprouts
3.2. In Vitro Antioxidant Capability
3.2.1. Determination of Total Phenolic Content by Folin–Ciocalteu’s Reagent Method
3.2.2. Determination of Total Anthocyanin Content
3.2.3. Determination of DPPH Radical Scavenging Effect
3.2.4. Determination of Ferric Reducing Antioxidant Power
3.3. Determination of Cell Viability in Various Cancer and Noncancer Cell Lines
3.4. Determination of Intracellular Hydrogen Peroxide Level in Jurkat Cells
3.5. Determination of Endogenous Glutathione Level in Jurkat Cells
3.6. Apoptosis Induction Effect
3.6.1. Apoptosis Induction According to Flow Cytometric Analysis
3.6.2. Caspase 3/7 Activity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klaunig, J.E.; Wang, Z. Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 2018, 7, 116–121. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxi. Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, R.; Pandey, M.; Bhaskaran, N.; MacLennan, G.T.; Fu, P.; Ponsky, L.E.; Gupta, S. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1: GSTP1. Mol. Carcinog. 2014, 53, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed. Res. Int. 2014, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significant. Mutat. Res. Rev. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Cantoni, O.; Tasinato, A.; Boscoboinik, D.; Azzi, A. Hydrogen peroxide-and fetal bovine serum- induced DNA synthesis in vascular smooth muscle cells: Positive and negative regulation by protein kinase C isoforms. BBA Mol. Cell Res. 1995, 1269, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil Ocimum basilicum L. plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Ayoub, I.M.; El-Shazly, M.; Lu, M.-C.; Singab, A.N.B. Antimicrobial and cytotoxic activities of the crude extracts of Dietes bicolor leaves, flowers and rhizomes. S. Afr. J. Bot. 2014, 95, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Baena Ruiz, R.; Salinas Hernández, P. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [Green Version]
- Blancquaert, D.; Van Daele, J.; Strobbe, S.; Kiekens, F.; Storozhenko, S.; De Steur, H.; Gellynck, X.; Lambert, W.; Stove, C.; Van Der Straeten, D. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat. Biotechnol. 2015, 33, 1076–1078. [Google Scholar] [CrossRef]
- Zhu, Q.; Zeng, D.; Yu, S.; Cui, C.; Li, J.; Li, H.; Chen, J.; Zhang, R.; Zhao, X.; Chen, L.; et al. From golden rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol Plant. 2018, 11, 1440–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, K.S.; Findley, T.O.; Northrup, H. Finding the genetic mechanisms of folate deficiency and neural tube defects-leaving no stone unturned. Am. J. Med. Genet. 2017, 173, 3042–3057. [Google Scholar] [CrossRef] [PubMed]
- Socha, D.S.; DeSouza, S.I.; Flagg, A.; Sekeres, M.; Rogers, H.J. Severe megaloblastic anemia: Vitamin deficiency and other causes. Cleve Clin J. Med. 2020, 87, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, P.; Liu, X.; Li, J.; He, S.; Chen, H.; Yuan, Y.; Qiu, G.; Yu, K.; Liu, K.; Jiang, J.; et al. Circulating folate concentrations and risk of coronary artery disease: A prospective cohort study in Chinese adults and a Mendelian randomization analysis. Am. J. Clin. Nutr. 2020, 111, 635–643. [Google Scholar] [CrossRef]
- Corrada, M.M.; Kawas, C.H.; Hallfrisch, J.; Muller, D.; Brookmeyer, R. Reduced risk of Alzheimer’s disease with high folate intake: The Baltimore Longitudinal Study of Aging. Alzheimers. Dement. 2005, 1, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Punshon, T.; Carey, A.-M.; Ricachenevsky, F.K.; Meharg, A.A. Elemental distribution in developing rice grains and the effect of flag-leaf arsenate exposure. Environ. Exp. Bot. 2018, 149, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crop. Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Asthir, B.; Mahajan, G. Variation in antioxidants, bioactive compounds and antioxidant capacity in germinated and ungerminated grains of ten rice cultivars. Rice Sci. 2017, 24, 349–359. [Google Scholar] [CrossRef]
- Tamprasit, K.; Weerapreeyakul, N.; Sutthanut, K.; Thukhammee, W.; Wattanathorn, J. Harvest age effect on phytochemical content of white and black glutinous rice cultivars. Molecules 2019, 24, 4432. [Google Scholar] [CrossRef] [Green Version]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B. Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Sci. Hortic. 2019, 250, 302–309. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 2014, 8, 147–156. [Google Scholar] [CrossRef]
- Khanthapok, P.; Muangprom, A.; Sukrong, S. Antioxidant activity and DNA protective properties of rice grass juices. Sci. Asia 2015, 41, 119. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 10. [Google Scholar] [CrossRef] [Green Version]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Al-Qassabi, J.S.A.; Weli, A.M.; Hossain, M.A. Comparison of total phenols content and antioxidant potential of peel extracts of local and imported lemons samples. Sustain. Chem. Pharm. 2018, 8, 71–75. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Khamphio, M.; Barusrux, S.; Weerapreeyakul, N. Sesamol induces mitochondrial apoptosis pathway in HCT116 human colon cancer cells via pro-oxidant effect. Life Sci. 2016, 158, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N. Simultaneous quantification of sulforaphene and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016, 201, 139–144. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Thumanu, K. Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomol. Ther. 2019, 27, 540–552. [Google Scholar] [CrossRef]
- Gerl, R. Apoptosis in the development and treatment of cancer. Carcinogenesis 2004, 26, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, F.; Li, J.; Xu, Y.; Wang, L.; Zhang, Y. Docetaxel down-regulates PD-1 expression via STAT3 in T lymphocytes. Clin. Lung Cancer 2018, 19, e675–e683. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Dinh, N.-T.; Skolnick, J.; Styczynski, M.P. Metabolomics identifies the intersection of phosphoethanolamine with menaquinone-triggered apoptosis in an in vitro model of leukemia. Mol. BioSyst. 2015, 11, 2406–2416. [Google Scholar] [CrossRef] [Green Version]
- Si, M.-S.; Imagawa, D.K.; Ji, P.; Wei, X.; Holm, B.; Kwok, J.; Lee, M.; Reitz, B.A.; Borie, D.C. Immunomodulatory effects of docetaxel on human lymphocytes. Invest. New Drugs 2003, 21, 281–290. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidayat, M.A.; Fitri, A.; Kuswandi, B. Scanometry as microplate reader for high throughput method based on DPPH dry reagent for antioxidant assay. Acta Pharm. Sin. B 2017, 7, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridge, G.; Rashid, S.; Martin, S.A. DNA mismatch repair and oxidative DNA damage: Implications for cancer biology and treatment. Cancers 2014, 6, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig. Med. Dosw. 2014, 13, 528–540. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Screening phytochemical content, antioxidant, antimicrobial and cytotoxic activities of Catharanthus roseus (L.) G. Don stem extract and its fractions. Biocatal. Agric. Biotechnol. 2018, 16, 405–411. [Google Scholar] [CrossRef]
- Deepika, S.; Selvaraj, C.I.; Roopan, S.M. Screening bioactivities of Caesalpinia pulcherrima L. swartz and cytotoxicity of extract synthesized silver nanoparticles on HCT116 cell line. Mater. Sci. Eng. C 2020, 106, 110279. [Google Scholar] [CrossRef]
- Mu, W.; Liu, L.-Z. Reactive oxygen species signaling in cancer development. React. Oxyg. Species 2017, 4, 251–265. [Google Scholar] [CrossRef]
- Matt, S.; Hofmann, T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell. Mol. Life Sci. 2016, 73, 2829–2850. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B. DNA damage-triggered apoptosis: Critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol. 2003, 66, 1547–1554. [Google Scholar] [CrossRef]
- Aljuhani, N.; Ismail, R.S.; El-Awady, M.S.; Hassan, M.H. Modulatory effects of perindopril on cisplatin-induced nephrotoxicity in mice: Implication of inflammatory cytokines and caspase-3 mediated apoptosis. Acta Pharm. 2020, 70, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, Y.; Nemoto, H.; Itoh, M.; Kosaka, H.; Morita, K. Fermented brown rice extract causes apoptotic death of human acute lymphoblastic leukemia cells via death receptor pathway. Appl. Biochem. Biotechnol. 2016, 178, 1599–1611. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Cordier, W.; Steenkamp, P.; Steenkamp, V. Extracts of Moringa oleifera lack cytotoxicity and attenuate oleic acid-induced steatosis in an in vitro HepG2 model. S. Afr. J. Bot. 2019, S0254629919301607. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. Flavonoids in cancer prevention. Anti Cancer Agents Med. Chem. 2012, 12, 836–851. [Google Scholar] [CrossRef] [PubMed]



| Age (Day), and Height of Rice Sprouts (cm) | % Yields (w/w Fresh Weight) | |
|---|---|---|
| BGR | 5–7 days, 5–7 cm | 8.69% 6 |
| 10–15 days, 10–12 cm | 13.30% 4 | |
| 20–25 days, 15–17 cm | 13.25% 5 | |
| RD6 | 5–7 days, 5–7 cm | 60.12% 2 |
| 10–15 days, 10–12 cm | 25.97% 3 | |
| 20–25 days, 15–17 cm | 63.50% 1 | |
| Test Compounds | Ages (days) | IC50 (μg/mL) ± SD |
|---|---|---|
| BGR | 5–7 | 271.2 ± 13.1 a |
| 10–15 | 238.8 ± 10.4 a | |
| 20–25 | 231.1 ± 12.9 a | |
| RD6 | 5–7 | 571.7 ± 17.4 b |
| 10–15 | 575.8 ± 38.2 b | |
| 20–25 | 636.7 ± 34.6 c | |
| Gallic acid | - | 2.24 ± 0.03 |
| Rice Cultivars | Ages (days) | FRAP Power (mmole) Per Weight in Gram of Extract | ||
|---|---|---|---|---|
| 100 μg/mL | 200 μg/mL | 1000 μg/mL | ||
| BGR | 5–7 | 0.79 ± 0.0051 cC | 0.84 ± 0.0068 cB | 1.28 ± 0.015 cA |
| 10–15 | 1.37 ± 0.017 aC | 1.86 ± 0.069 aB | 2.22 ± 0.014 aA | |
| 20–25 | 1.28 ± 0.011 bB | 1.61 ± 0.115 bA | 1.58 ± 0.0302 bA | |
| RD6 | 5–7 | 0.73 ± 0.004 eC | 0.76 ± 0.0049 dB | 0.93 ± 0.0083 fA |
| 10–15 | 0.77 ± 0.016 dC | 0.85 ± 0.061 cB | 1.21 ± 0.016 dA | |
| 20–25 | 0.73 ± 0.019 eB | 0.76 ± 0.018 dB | 1.03 ± 0.019 eA | |
| Time | Compounds | Ages (Days) | IC50 (µg/mL) ± SD (Selectivity Index) | ||||
|---|---|---|---|---|---|---|---|
| Jurkat | HepG2 | HCT116 | SK-MEL-2 | Vero | |||
| Cisplatin | 61.8 ± 1.3 a | 65.1 ± 0.6 a | 25.8 ± 0.5 a | 27.7 ± 0.5 a | 121.5 ± 1.6 a | ||
| 24 h | BGR | 5–7 | 206.2 ± 15.1 aA (4.8) | Inactive bC | 806.2 ± 16.0 cB (1.2) | Inactive bC | Inactive bC |
| 10–15 | 261.9 ± 6.6 bA (3.8) | Inactive bC | 513.4 ± 18.3 bB (1.9) | Inactive bC | Inactive bC | ||
| 20–25 | 438.6 ± 11.6 dB (2.3) | Inactive bC | 426.4 ± 6.1 aA (2.3) | Inactive bC | Inactive bC | ||
| RD6 | 5–7 | Inactive eA | Inactive bA | Inactive dA | Inactive bA | Inactive bA | |
| 10–15 | 310.2 ± 28.0 cA (3.2) | Inactive bB | Inactive dB | Inactive bB | Inactive bB | ||
| 20–25 | Inactive eA | Inactive bA | Inactive dA | Inactive bA | Inactive bA | ||
| 48 h | BGR | 5–7 | 67.62 ± 6.8 aA (14.8) | 323.7 ± 18.5 aB (3.1) | 439.4 ± 22.7 cC (2.3) | Inactive dD | Inactive aD |
| 10–15 | 166.7 ± 13.2 bA (6.0) | Inactive bC | 391.0 ± 54.3 ab,B (2.6) | Inactive dC | Inactive aC | ||
| 20–25 | 136.4 ± 4.9 ab,A (7.3) | 326.5 ± 19.0 aB (3.1) | 379.0 ± 11.0 aB (2.6) | Inactive dD | Inactive aD | ||
| RD6 | 5–7 | Inactive dA | Inactive bA | Inactive dA | Inactive aA | Inactive aA | |
| 10–15 | 508.2 ± 20.8 cA (2.0) | Inactive bB | Inactive dB | 677.8 ± 35.7 bA (1.5) | Inactive aB | ||
| 20–25 | Inactive dA | Inactive bA | Inactive dA | Inactive aA | Inactive aA | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, V.; Pocasap, P.; Sutthanut, K.; Sethabouppha, B.; Thukhammee, W.; Wattanathorn, J.; Weerapreeyakul, N. Effect of Harvest Age on Total Phenolic, Total Anthocyanin Content, Bioactive Antioxidant Capacity and Antiproliferation of Black and White Glutinous Rice Sprouts. Appl. Sci. 2020, 10, 7051. https://doi.org/10.3390/app10207051
So V, Pocasap P, Sutthanut K, Sethabouppha B, Thukhammee W, Wattanathorn J, Weerapreeyakul N. Effect of Harvest Age on Total Phenolic, Total Anthocyanin Content, Bioactive Antioxidant Capacity and Antiproliferation of Black and White Glutinous Rice Sprouts. Applied Sciences. 2020; 10(20):7051. https://doi.org/10.3390/app10207051
Chicago/Turabian StyleSo, Visessakseth, Piman Pocasap, Khaetthareeya Sutthanut, Benjabhorn Sethabouppha, Wipawee Thukhammee, Jintanaporn Wattanathorn, and Natthida Weerapreeyakul. 2020. "Effect of Harvest Age on Total Phenolic, Total Anthocyanin Content, Bioactive Antioxidant Capacity and Antiproliferation of Black and White Glutinous Rice Sprouts" Applied Sciences 10, no. 20: 7051. https://doi.org/10.3390/app10207051