Fluorene–Triphenylamine-Based Bipolar Materials: Fluorescent Emitter and Host for Yellow Phosphorescent OLEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of N1-(9,9-diphenyl-9H-fluoren-2-yl)-N4,N4-diphenylbenzene-1,4-diamine (3)
2.4. Characterization of N1-(9,9-diphenyl-9H-fluoren-2-yl)-N4,N4-diphenylbenzene-1,4-diamine (3)
2.5. Synthesis of 2-chloro-4,6-diphenylpyrimidine (6)
2.6. Characterization of 2-chloro-4,6-diphenylpyrimidine (6)
2.7. Synthesis of N1-(9,9-diphenyl-9H-fluoren-2-yl)-N1-(4,6-diphenylpyrimidin-2-yl)-N4,N4 diphenylbenzene-1,4-diamine (FLU-TPA/PYR)
2.8. Characterization of N1-(9,9-diphenyl-9H-fluoren-2-yl)-N1-(4,6-diphenylpyrimidin-2-yl)-N4,N4 diphenylbenzene-1,4-diamine (FLU-TPA/PYR)
2.9. Synthesis of N1-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-N1-(9,9-diphenyl-9H-fluoren-2-yl)-N4,N4-diphenylbenzene-1,4-diamine (FLU-TPA/TRZ)
2.10. Characterization of N1-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-N1-(9,9-diphenyl-9H-fluoren-2-yl)-N4,N4-diphenylbenzene-1,4-diamine (FLU-TPA/TRZ)
2.11. OLED Device Fabrication
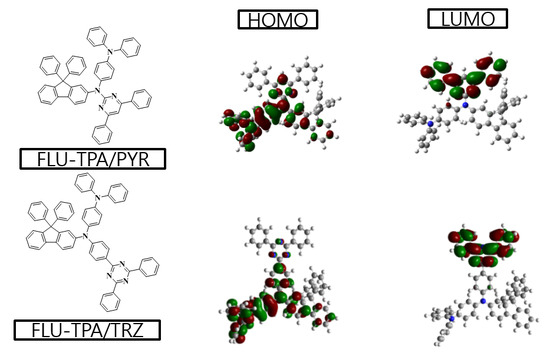
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ito, T.; Sasabe, H.; Nagai, Y.; Watanabe, Y.; Onuma, N.; Kido, J. A series of dibenzofuran-based n-type exciplex host partners realizing high-efficiency and stable deep-red phosphorescent oleds. Chem. A Eur. J. 2019, 25, 7308–7314. [Google Scholar] [CrossRef]
- Kido, J.; Kimura, M.; Nagai, K. Multilayer white light-emitting organic electroluminescent device. Science 1995, 267, 1332–1334. [Google Scholar] [CrossRef] [Green Version]
- Braveenth, R.; Bae, H.W.; Ko, I.J.; Qiong, W.; Nguyen, Q.P.B.; Jayashantha, P.G.S.; Kwon, J.H.; Chai, K.Y. Thermally stable efficient hole transporting materials based on carbazole and triphenylamine core for red phosphorescent oleds. Org. Electron. 2017, 51, 463–470. [Google Scholar] [CrossRef]
- Promarak, V.; Ichikawa, M.; Meunmart, D.; Sudyoadsuk, T.; Saengsuwan, S.; Keawin, T. Synthesis and properties of stable amorphous hole-transporting molecules for electroluminescent devices. Tetrahedron Lett. 2006, 47, 8949–8952. [Google Scholar] [CrossRef]
- Kim, B.M.; Nguyen, Q.P.B.; Fan, J.G.; Kim, M.J.; Braveenth, R.; Kim, G.W.; Kwon, J.H.; Chai, K.Y. Novel star-shaped hole-transporting materials based on triphenylamine cores end-capped with carbazole and triarylamine derivatives for use in oleds. Bull. Korean Chem. Soc. 2015, 36, 1303–1306. [Google Scholar] [CrossRef]
- Kochapradist, P.; Prachumrak, N.; Tarsang, R.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Multi-triphenylamine-substituted carbazoles: Synthesis, characterization, properties and applications as hole-transporting materials. Tetrahedron Lett. 2013, 54, 3683–3687. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, C.-H.; Lee, P.-H.; Lin, H.-Y.; Leung, M.-k.; Chiu, T.-L.; Lin, C.-F. Blue organic light-emitting diodes: Current status, challenges, and future outlook. J. Mater. Chem. C 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Xiao, P.; Dong, T.; Xie, J.; Luo, D.; Yuan, J.; Liu, B. Emergence of white organic light-emitting diodes based on thermally activated delayed fluorescence. Appl. Sci. 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Braveenth, R.; Chai, K.Y. Triazine-acceptor-based green thermally activated delayed fluorescence materials for organic light-emitting diodes. Materials 2019, 12, 2646. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Dai, X.; Yao, F.; Wei, X.; Cao, J.; Jhun, C. Efficient white phosphorescent organic light-emitting diodes using ultrathin emissive layers (<1 nm). Sci. Rep. 2018, 8, 6068. [Google Scholar]
- Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 2015, 6, 8476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrade, B. 5—Phosphorescent oleds for solid-state lighting. In Organic Light-Emitting Diodes (Oleds); Buckley, A., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 143–169. [Google Scholar]
- Chen, J.-X.; Tao, W.-W.; Xiao, Y.-F.; Tian, S.; Chen, W.-C.; Wang, K.; Yu, J.; Geng, F.-X.; Zhang, X.-H.; Lee, C.-S. Isomeric thermally activated delayed fluorescence emitters based on indolo[2,3-b]acridine electron-donor: A compromising optimization for efficient orange–red organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 2898–2904. [Google Scholar] [CrossRef]
- Fukagawa, H.; Shimizu, T.; Kamada, T.; Kiribayashi, Y.; Osada, Y.; Hasegawa, M.; Morii, K.; Yamamoto, T. Highly efficient and stable phosphorescent organic light-emitting diodes utilizing reverse intersystem crossing of the host material. Adv. Opt. Mater. 2014, 2, 1070–1075. [Google Scholar] [CrossRef]
- Zhan, G.; Liu, Z.; Bian, Z.; Huang, C. Recent advances in organic light-emitting diodes based on pure organic room temperature phosphorescence materials. Front. Chem. 2019, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Hoffmann, S.T.; Heinemeyer, U.; Münster, I.; Köhler, A.; Strohriegl, P. Triazine based bipolar host materials for blue phosphorescent oleds. Chem. Mater. 2013, 25, 3758–3765. [Google Scholar] [CrossRef]
- Cui, L.-S.; Kim, J.U.; Nomura, H.; Nakanotani, H.; Adachi, C. Benzimidazobenzothiazole-based bipolar hosts to harvest nearly all of the excitons from blue delayed fluorescence and phosphorescent organic light-emitting diodes. Angew. Chem. Int. Ed. 2016, 55, 6864–6868. [Google Scholar] [CrossRef]
- Mori, K.; Goumans, T.P.M.; van Lenthe, E.; Wang, F. Predicting phosphorescent lifetimes and zero-field splitting of organometallic complexes with time-dependent density functional theory including spin–orbit coupling. Phys. Chem. Chem. Phys. 2014, 16, 14523–14530. [Google Scholar] [CrossRef]
- Chou, P.-T.; Chi, Y. Osmium- and ruthenium-based phosphorescent materials: Design, photophysics and utilization in oled fabrication. Eur. J. Inorg. Chem. 2006, 2006, 3319–3332. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Ho, C.-L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. [Google Scholar] [CrossRef]
- Kawamura, Y.; Goushi, K.; Brooks, J.; Brown, J.J.; Sasabe, H.; Adachi, C. 100% phosphorescence quantum efficiency of ir(iii) complexes in organic semiconductor films. Appl. Phys. Lett. 2005, 86, 071104. [Google Scholar] [CrossRef]
- Shih, P.-I.; Chiang, C.-L.; Dixit, A.K.; Chen, C.-K.; Yuan, M.-C.; Lee, R.-Y.; Chen, C.-T.; Diau, E.W.-G.; Shu, C.-F. Novel carbazole/fluorene hybrids: Host materials for blue phosphorescent oleds. Org. Lett. 2006, 8, 2799–2802. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Robinson, M.R.; Ostrowski, J.C.; Moses, D.; Bazan, G.C.; Heeger, A.J. High-efficiency polymer-based electrophosphorescent devices. Adv. Mater. 2002, 14, 581–585. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Y.; Yang, C.; Cao, Y.; Tao, Y.; Qin, J.; Ma, D. A fully diarylmethylene-bridged triphenylamine derivative as novel host for highly efficient green phosphorescent oleds. Org. Lett. 2009, 11, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, K.; Zhao, G.; Wei, J.; Duan, J.; Xia, M.; Jiang, W.; Sun, Y. Spatial separation of a tadf sensitizer and fluorescent emitter with a core-dendron system to block the energy loss in deep blue organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 11005–11013. [Google Scholar] [CrossRef]
- Song, D.; Zhao, S.; Luo, Y.; Aziz, H. Causes of efficiency roll-off in phosphorescent organic light emitting devices: Triplet-triplet annihilation versus triplet-polaron quenching. Appl. Phys. Lett. 2010, 97, 243304. [Google Scholar] [CrossRef]
- Serevičius, T.; Komskis, R.; Adomėnas, P.; Adomėnienė, O.; Kreiza, G.; Jankauskas, V.; Kazlauskas, K.; Miasojedovas, A.N.; Jankus, V.; Monkman, A.; et al. Triplet–triplet annihilation in 9, 10-diphenylanthracene derivatives: The role of intersystem crossing and exciton diffusion. J. Phys. Chem. C 2017, 121, 8515–8524. [Google Scholar] [CrossRef]
- Flügge, H.; Rohr, A.; Döring, S.; Fléchon, C.; Wallesch, M.; Zink, D.; Seeser, J.; Leganés, J.; Sauer, T.; Rabe, T.; et al. Reduced Concentration Quenching in a Tadf-Type Copper(I)-Emitter; SPIE: Bellingham, WA, USA, 2015; Volume 9566. [Google Scholar]
- Wang, Z.; Li, M.; Gan, L.; Cai, X.; Li, B.; Chen, D.; Su, S.-J. Predicting operational stability for organic light-emitting diodes with exciplex cohosts. Adv. Sci. 2019, 6, 1802246. [Google Scholar] [CrossRef]
- Huang, R.; Kukhta, N.A.; Ward, J.S.; Danos, A.; Batsanov, A.S.; Bryce, M.R.; Dias, F.B. Balancing charge-transfer strength and triplet states for deep-blue thermally activated delayed fluorescence with an unconventional electron rich dibenzothiophene acceptor. J. Mater. Chem. C 2019, 7, 13224–13234. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, U.; Singh, J. Study of processes of reverse intersystem crossing (RISC) and thermally activated delayed fluorescence (TADF) in organic light emitting diodes (OLEDS). Org. Electron. 2018, 59, 121–124. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Li, Y.; Qiu, Y.; Duan, L. Understanding the operational lifetime expansion methods of thermally activated delayed fluorescence sensitized oleds: A combined study of charge trapping and exciton dynamics. Mater. Chem. Front. 2019, 3, 1181–1191. [Google Scholar] [CrossRef]
- Hosokai, T.; Matsuzaki, H.; Nakanotani, H.; Tokumaru, K.; Tsutsui, T.; Furube, A.; Nasu, K.; Nomura, H.; Yahiro, M.; Adachi, C. Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states. Sci. Adv. 2017, 3, e1603282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-X.; Tao, W.-W.; Wang, K.; Zheng, C.-J.; Liu, W.; Li, X.; Ou, X.-M.; Zhang, X.-H. Highly efficient thermally activated delayed fluorescence emitters based on novel indolo[2,3-b]acridine electron-donor. Org. Electron. 2018, 57, 327–334. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Ward, J.S.; Bryce, M.R.; Monkman, A.P. Using guest–host interactions to optimize the efficiency of tadf oleds. J. Phys. Chem. Lett. 2016, 7, 3341–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, W.S.; Park, T.J.; Kim, S.Y.; Pode, R.; Jang, J.; Kwon, J.H. Ideal host and guest system in phosphorescent oleds. Org. Electron. 2009, 10, 240–246. [Google Scholar] [CrossRef]
- Lee, H.L.; Lee, K.H.; Lee, J.Y. Indoloindole as a new building block of a hole transport type host for stable operation in phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 5988–5994. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.Y. Exciplex hosts for blue phosphorescent organic light-emitting diodes. J. Inf. Disp. 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Koo, H.; Kwon, O.; Jae Park, Y.; Choi, H.; Lee, K.; Ahn, B.; Min Park, Y. The role of charge balance and excited state levels on device performance of exciplex-based phosphorescent organic light emitting diodes. Sci. Rep. 2017, 7, 11995. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Lee, J.-H.; Chen, C.-H.; Chen, P.-H.; Wang, P.-S.; Lin, C.-E.; Lin, B.-Y.; Lan, Y.-H.; Hsieh, Y.-H.; Huang, J.-J.; et al. Carrier transport and recombination mechanism in blue phosphorescent organic light-emitting diode with hosts consisting of cabazole- and triazole-moiety. Sci. Rep. 2019, 9, 3654. [Google Scholar] [CrossRef]
- Lin, M.-S.; Yang, S.-J.; Chang, H.-W.; Huang, Y.-H.; Tsai, Y.-T.; Wu, C.-C.; Chou, S.-H.; Mondal, E.; Wong, K.-T. Incorporation of a cn group into mcp: A new bipolar host material for highly efficient blue and white electrophosphorescent devices. J. Mater. Chem. 2012, 22, 16114–16120. [Google Scholar] [CrossRef]
- Kang, J.S.; Hong, T.R.; Kim, H.J.; Son, Y.H.; Lampande, R.; Kang, B.Y.; Lee, C.; Bin, J.-K.; Lee, B.S.; Yang, J.H.; et al. High-performance bipolar host materials for blue tadf devices with excellent external quantum efficiencies. J. Mater. Chem. C 2016, 4, 4512–4520. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, Y.; Cheng, J.; Li, J. A cbp derivative as bipolar host for performance enhancement in phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2013, 1, 757–764. [Google Scholar] [CrossRef]
- Maasoumi, F.; Jansen-van Vuuren, R.D.; Shaw, P.E.; Puttock, E.V.; Nagiri, R.C.R.; McEwan, J.A.; Bown, M.; O’Connell, J.L.; Dunn, C.J.; Burn, P.L.; et al. An external quantum efficiency of >20% from solution-processed poly(dendrimer) organic light-emitting diodes. Npj Flex. Electron. 2018, 2, 27. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Z. Recent applications of interfacial exciplex as ideal host of power-efficient oleds. Front. Chem. 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Sun, X.; Hu, Y.; Mu, W.; Sun, Y.; Zhang, D.; Su, Z.; Chu, B.; Cui, Z. Blended host ink for solution processing high performance phosphorescent oleds. Sci. Rep. 2019, 9, 6845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.W.; Lee, C. Effect of host materials on eelectrophosphorescence properties of ptoep-doped organic light-emitting diodes. J. Inf. Disp. 2007, 8, 15–19. [Google Scholar] [CrossRef]
- Ihn, S.-G.; Lee, N.; Jeon, S.O.; Sim, M.; Kang, H.; Jung, Y.; Huh, D.H.; Son, Y.M.; Lee, S.Y.; Numata, M.; et al. An alternative host material for long-lifespan blue organic light-emitting diodes using thermally activated delayed fluorescence. Adv. Sci. 2017, 4, 1600502. [Google Scholar] [CrossRef]
- Kim, G.W.; Bae, H.W.; Lampande, R.; Ko, I.J.; Park, J.H.; Lee, C.Y.; Kwon, J.H. Highly efficient single-stack hybrid cool white oled utilizing blue thermally activated delayed fluorescent and yellow phosphorescent emitters. Sci. Rep. 2018, 8, 16263. [Google Scholar] [CrossRef]
- Braveenth, R.; Ahn, D.H.; Han, J.-H.; Moon, J.S.; Kim, S.W.; Lee, H.; Qiong, W.; Kwon, J.H.; Chai, K.Y. Utilizing triazine/pyrimidine acceptor and carbazole-triphenylamine donor based bipolar novel host materials for highly luminescent green phosphorescent oleds with lower efficiency roll-off. Dye. Pigment. 2018, 157, 377–384. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, C.W.; Lee, J.Y. High triplet energy host materials for blue phosphorescent organic light-emitting diodes derived from carbazole modified orthophenylene. J. Mater. Chem. C 2014, 2, 7256–7263. [Google Scholar] [CrossRef]
- Godumala, M.; Choi, S.; Kim, S.K.; Kim, S.W.; Kwon, J.H.; Cho, M.J.; Choi, D.H. Highly efficient bipolar host materials towards solution-processable blue and green thermally activated delayed fluorescence organic light emitting diodes. J. Mater. Chem. C 2018, 6, 10000–10009. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, Z.; Qu, W.; Yang, T.; Li, J.; Wang, L. Rational design of quinoxaline-based bipolar host materials for highly efficient red phosphorescent organic light-emitting diodes. Rsc Adv. 2019, 9, 10789–10795. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.-R.; Lee, C.W.; Gong, M.-S. Bipolar host material for phosphorescent oleds based on 2, 7-diazacarbazole as a new electron-transporting unit. Bull. Korean Chem. Soc. 2017, 38, 1016–1022. [Google Scholar] [CrossRef]
- Vadagaonkar, K.S.; Yang, C.-J.; Zeng, W.-H.; Chen, J.-H.; Patil, B.N.; Chetti, P.; Chen, L.-Y.; Chaskar, A.C. Triazolopyridine hybrids as bipolar host materials for green phosphorescent organic light-emitting diodes (oleds). Dyes Pigment. 2019, 160, 301–314. [Google Scholar] [CrossRef]
- Chang, C.-H.; Kuo, M.-C.; Lin, W.-C.; Chen, Y.-T.; Wong, K.-T.; Chou, S.-H.; Mondal, E.; Kwong, R.C.; Xia, S.; Nakagawa, T.; et al. A dicarbazole–triazine hybrid bipolar host material for highly efficient green phosphorescent oleds. J. Mater. Chem. 2012, 22, 3832–3838. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, D.; Wang, F.; Zhang, S. Bipolar host materials for high-efficiency blue phosphorescent and delayed-fluorescence oleds. J. Mater. Chem. C 2015, 3, 12529–12538. [Google Scholar] [CrossRef]












| Bipolar Materials | FLU-TPA/PYR | FLU-TPA/TRZ |
|---|---|---|
| Tg (°C) | 153 | 147 |
| Td (°C) | 426 | 478 |
| UV-Vis (nm) | 410 | 412 |
| PL (nm) | 548 | 606 |
| Band gap (eV) | 2.41 | 2.49 |
| Triplet energy (eV) | 2.57 | 2.77 |
| Highest occupied molecular orbital (HOMO) (eV) | 5.27 | 5.35 |
| Lowest occupied molecular orbital (LUMO) (eV) | 2.86 | 2.86 |
| Device Characteristics | FLU-TPA/PYR (Yellow Device) | FLU-TPA/TRZ (Yellow Device) | FLU-TPA/PYR (Non-Doped) | FLU-TPA/TRZ (Non-Doped) |
|---|---|---|---|---|
| Turn-On Voltage (V) | 5.0 | 5.0 | 6.5 | 5.0 |
| Current Efficiency (cd/A) | 21.70 | 18.72 | 1.27 | 10.30 |
| Power Efficiency (lm/W) | 13.64 | 11.76 | 0.61 | 6.47 |
| EQE (%) | 7.75 | 6.44 | 0.53 | 3.57 |
| CIE (x,y) | 0.49,0.50 | 0.49,0.50 | 0.41,0.51 | 0.41,0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braveenth, R.; Jung, H.; Kim, K.; Kim, B.M.; Bae, I.-J.; Kim, M.; Chai, K.Y. Fluorene–Triphenylamine-Based Bipolar Materials: Fluorescent Emitter and Host for Yellow Phosphorescent OLEDs. Appl. Sci. 2020, 10, 519. https://doi.org/10.3390/app10020519
Braveenth R, Jung H, Kim K, Kim BM, Bae I-J, Kim M, Chai KY. Fluorene–Triphenylamine-Based Bipolar Materials: Fluorescent Emitter and Host for Yellow Phosphorescent OLEDs. Applied Sciences. 2020; 10(2):519. https://doi.org/10.3390/app10020519
Chicago/Turabian StyleBraveenth, Ramanaskanda, Hasu Jung, Keunhwa Kim, Bo Mi Kim, Il-Ji Bae, Miyoung Kim, and Kyu Yun Chai. 2020. "Fluorene–Triphenylamine-Based Bipolar Materials: Fluorescent Emitter and Host for Yellow Phosphorescent OLEDs" Applied Sciences 10, no. 2: 519. https://doi.org/10.3390/app10020519