Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake
Abstract
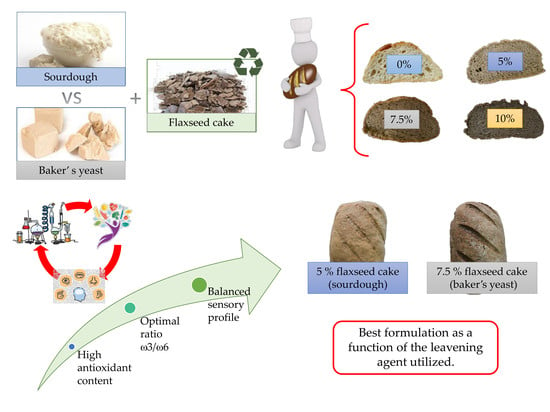
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Material
2.3. Bread Preparation
2.4. Physicochemical Characterization and Volatile Organic Compounds Profile
2.5. Color Determination
2.6. Nutraceutical Characterization of Breads
2.6.1. Extract Preparation
2.6.2. Total Phenol Evaluation
2.6.3. Total Flavonoid Evaluation
2.6.4. Determination of Anti-Radical Activity
2.6.5. Fatty Acid Profile Characterization
2.7. Sensory Characterization (Crust and Crumb)
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Color Determination
3.3. Nutraceutical Characterization of Bread
3.4. Volatiles Bouquet in the Headspace Emissions of the Cooked Breads
3.5. Sensorial Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mondal, A.; Datta, A.K. Bread baking—A review. J. Food Eng. 2008, 86, 465–474. [Google Scholar] [CrossRef]
- Katina, K.; Heiniö, R.-L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Zinnai, A. Effect of the baking process on artisanal sourdough bread-making: A technological and sensory evaluation. Agrochimica 2016, 60, 222–234. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Mercier, S.; Villeneuve, S.; Moresoli, C.; Mondor, M.; Marcos, B.; Power, K.A. Flaxseed-enriched cereal-based products: A review of the impact of processing conditions. Compr. Rev. Food Sci. Food Saf. 2014, 13, 400–412. [Google Scholar] [CrossRef]
- De Lamo, B.; Gómez, M. Bread enrichment with oilseeds. A review. Foods 2018, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Collado-Fernández, M. BREAD|Breadmaking Processes. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 627–634. [Google Scholar]
- Struyf, N.; Van der Maelen, E.; Hemdane, S.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Bread Dough and Baker’s Yeast: An Uplifting Synergy. Compr. Rev. Food Sci. Food Saf. 2017, 16, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Carbonetto, B.; Ramsayer, J.; Nidelet, T.; Legrand, J.; Sicard, D. Bakery yeasts, a new model for studies in ecology and evolution. Yeast 2018, 35, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Gélinas, P. Fermentation Control in Baker’s Yeast Production: Mapping Patents. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1141–1164. [Google Scholar] [CrossRef]
- Gänzle, M.G. BREAD|Sourdough Bread. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 309–315. [Google Scholar]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; di Palo, D.M.; Lorusso, M.P.; de Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [Green Version]
- Nionelli, L.; Rizzello, C. Sourdough-Based Biotechnologies for the Production of Gluten-Free Foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef]
- Ravishankar, R. Advances in Food Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-86455-5. [Google Scholar]
- Cardoso, R.V.C.; Fernandes, Â.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Flour fortification for nutritional and health improvement: A review. Food Res. Int. 2019, 125, 108576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meybodi, N.M.; Mirmoghtadaie, L.; Sheidaei, Z.; Mortazavian, A.M. Wheat Bread: Potential Approach to Fortify its Lysine Content. Curr. Nutr. Food Sci. 2019, 15, 630–637. [Google Scholar] [CrossRef]
- Betoret, E.; Rosell, C.M. Enrichment of bread with fruits and vegetables: Trends and strategies to increase functionality. Cereal Chem. 2020, 97, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.S.; Talou, T.; Straumite, E.; Sabovics, M.; Kruma, Z.; Saad, Z.; Hijazi, A.; Merah, O. Protein bread fortification with cumin and caraway seeds and by-product flour. Foods 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ. Sci. 2018, 30, 278–282. [Google Scholar] [CrossRef]
- Turfani, V.; Narducci, V.; Durazzo, A.; Galli, V.; Carcea, M. Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT Food Sci. Technol. 2017, 78, 361–366. [Google Scholar] [CrossRef]
- Świeca, M.; Sȩczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves—The influence of protein-phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Boukid, F.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for bread fortification: A necessity or a choice? Trends Food Sci. Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Dziki, D.; Anders, A.; Gawlik-Dziki, U. Antioxidant, nutritional and functional characteristics of wheat bread enriched with ground flaxseed hulls. Food Chem. 2017, 214, 32–38. [Google Scholar] [CrossRef]
- Sgherri, C.; Micaelli, F.; Andreoni, N.; Baldanzi, M.; Ranieri, A. Retention of phenolic compounds and antioxidant properties in potato bread obtained from a dough enriched with a powder from the purple cv. Vitelotte. Agrochimica 2016, 60, 312–328. [Google Scholar] [CrossRef]
- Dziki, D.; Rózyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilek, T.Z.T.; Kairuaman, N.A.; Ahmad, F.; Wahab, R.A.; Zamri, A.I.; Mahmood, A. Development of white bread fortified with calcium derived from Eggshell powder. Malays. Appl. Biol. 2018, 47, 29–39. [Google Scholar]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Nadathur, S.R.; Wanasundara, J.P.; Scanlin, L. Proteins in the Diet. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–19. ISBN 9780128027783. [Google Scholar]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Anjum, F.M.; Alamri, M.S. Fortification of pan bread with healthy flaxseed. Aust. J. Basic Appl. Sci. 2011, 5, 978–983. [Google Scholar]
- Moraes, É.A.; Dantas, M.I.D.S.; Morais, D.D.C.; Silva, C.O.D.; de Castro, F.A.F.; Martino, H.S.D.; Ribeiro, S.M.R. Sensory evaluation and nutritional value of cakes prepared with whole flaxseed flour. Ciência Tecnol. Aliment. 2010, 30, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Roozegar, M.H.; Shahedi, M.; Keramet, J.; Hamdami, N.; Roshanak, S. Effect of coated and uncoated ground flaxseed addition on rheological, physical and sensory properties of Taftoon bread. J. Food Sci. Technol. 2015, 52, 5102–5110. [Google Scholar] [CrossRef] [Green Version]
- Lipilina, E.; Ganji, V. Incorporation of ground flaxseed into bakery products and its effect on sensory and nutritional characteristics—A pilot study. J. Foodserv. 2009, 20, 52–59. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1–9. [Google Scholar]
- De Silva, S.F.; Alcorn, J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menteş, Ö.; Bakkalbaşi, E.; Ercan, R. Effect of the use of ground flaxseed on quality and chemical composition of bread. Food Sci. Technol. Int. 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Katina, K. Sourdough: A Tool for the Improved Flavour, Texture and Shelf-Life of Wheat Bread; VTT Technical Research Centre of Finland: Vuorimiehentie, Finland, 2005; ISBN 12350621. [Google Scholar]
- Gobbetti, M.; Gänzle, M. Handbook on Sourdough Biotechnology; Springer: Boston, MA, USA, 2013; ISBN 9781461454250. [Google Scholar]
- Heitmann, M.; Zannini, E.; Arendt, E. Impact of Saccharomyces cerevisiae metabolites produced during fermentation on bread quality parameters: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1152–1164. [Google Scholar] [CrossRef]
- Heitmann, M.; Zannini, E.; Arendt, E.K. Impact of different beer yeasts on wheat dough and bread quality parameters. J. Cereal Sci. 2015, 63, 49–56. [Google Scholar] [CrossRef]
- Method 44-15.02 Moisture—Air-Oven Methods. In AACC Approved Methodof Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999.
- Method 02-52.01. Hydrogen-Ion Activity (pH)—Electrometric Method. In AACC Approved Methodof Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1961.
- Method 02-01.02 Fat Acidity—General Method. In AACC Approved Methodof Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999.
- Gelinas, P.; Audet, J.; Lachance, O.; Vachon, M. Fermented Dairy Ingredients for Bread: Effects on Dough Rheology and Bread Characteristics’. Cereal Chem. 1995, 72, 151–154. [Google Scholar]
- Beutler, H.O. Ethanol. In Methods of Enzymatic Analysis; Bergmeyer, H.-U., Ed.; VCH Publisher (UK) Ltd.: Cambridge, UK, 1988; pp. 598–606. [Google Scholar]
- Noll, F. L-(+)-lactate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 582–588. [Google Scholar]
- Gawehn, K. D-(-)-lactate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 588–592. [Google Scholar]
- Beutler, H.O. Determination with Acetyl-CoA Synthetase. In Methods of Enzymatic Analysis; Bermeyer, H.-U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 639–645. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Macaluso, M.; Nari, A.; Quartacci, M.F.; Sgherri, C.; Flamini, G.; Taglieri, I.; Ascrizzi, R.; Andrich, G.; et al. Preliminary Results About the Use of Argon and Carbon Dioxide in the Extra Virgin Olive Oil (EVOO) Storage to Extend Oil Shelf Life: Chemical and Sensorial Point of View. Eur. J. Lipid Sci. Technol. 2018, 120, 1800156. [Google Scholar] [CrossRef]
- Venturi, F.; Andrich, G.; Sanmartin, C.; Scalabrelli, G.; Ferroni, G.; Zinnai, A. The expression of a full-bodied red wine as a function of the characteristics of the glass utilized for the tasting. CYTA J. Food 2014, 12, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Elía, M. A procedure for sensory evaluation of bread: Protocol developed by a trained panel. J. Sens. Stud. 2011, 26, 269–277. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hort, J.; Hollowood, T. Descriptive analysis in Sensory Evaluation, 1st ed.; Kemp, S.E., Hort, J., Hollowood, T., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; ISBN 9781118991671. [Google Scholar]
- Sánchez-Pardo, M.E.; Blancas-Nápoles, J.A.; Vázquez-Landaverde, P.A.; Nari, A.; Taglieri, I.; Ortiz-Moreno, A.; Mayorga-Reyes, L.; Sanmartin, C.; Bermúdez-Humarán, L.G.; Torres-Maravilla, E. The use of Mexican xaxtle as leavening agent in Italian straight dough bread making to produce pulque bread. Agrochimica 2016, 60, 329–342. [Google Scholar] [CrossRef]
- Barros, M.; Fleuri, L.F.; MacEdo, G.A. Seed lipases: Sources, applications and properties—A review. Braz. J. Chem. Eng. 2010, 27, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y.; Lai, O.M. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron de fi ciency anemia: A comprehensive review on iron absorption, bioavailability and emerging food forti fi cation approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Guo, X.; Shi, L.; Yang, S.; Yang, R.; Dai, X.; Zhang, T.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Effect of sea-buckthorn pulp and flaxseed residues on quality and shelf life of bread. Food Funct. 2019, 10, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The possibility of using by-products from the flaxseed industry for functional bread production. LWT 2020, 118, 108860. [Google Scholar] [CrossRef]
- Katare, C.; Saxena, S.; Agrawal, S.; Prasad, G. Flax Seed: A Potential Medicinal Food. J. Nutr. Food Sci. 2012, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Benítez, V.; Esteban, R.M.; Moniz, E.; Casado, N.; Aguilera, Y.; Mollá, E. Breads fortified with wholegrain cereals and seeds as source of antioxidant dietary fibre and other bioactive compounds. J. Cereal Sci. 2018, 82, 113–120. [Google Scholar] [CrossRef]
- de Aguiar, A.C.; Boroski, M.; Monteiro, A.R.G.; de Souza, N.E.; Visentainer, J.V. Enrichment of whole wheat flaxseed bread with flaxseed oil. J. Food Process. Preserv. 2011, 35, 605–609. [Google Scholar] [CrossRef]
- Kaur, P.; Waghmare, R.; Kumar, V.; Rasane, P.; Kaur, S.; Gat, Y. Recent advances in utilization of flaxseed as potential source for value addition. OCL Oilseeds Fats Crop. Lipids 2018, 25, A304. [Google Scholar] [CrossRef] [Green Version]
- Newton, I.; Snyder, D. Nutritional aspects of long chain omega-3 fatty acids and their use in bread enrichment. Cereal Food World 1997, 42, 126–131. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Choi, J.; Kim, Y.W.; Ryu, K.W.; Kim, Y.; Kim, J. Dietary n-3 and n-6 polyunsaturated fatty acids, the FADS gene, and the risk of gastric cancer in a Korean population. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Li, J. Physicochemical properties of steamed bread fortified with ground linseed (Linum usitatissimum). Int. J. Food Sci. Technol. 2019, 54, 1670–1676. [Google Scholar] [CrossRef]
- Zhu, F. Influence of ingredients and chemical components on the quality of Chinese steamed bread. Food Chem. 2014, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.D.; Davis, S.F. The effect of soya flour and flaxseed as a partial replacement for bread flour in yeast bread. Int. J. Food Sci. Technol. 2006, 41, 95–101. [Google Scholar] [CrossRef]
- Marpalle, P.; Sonawane, S.K.; LeBlanc, J.G.; Arya, S.S. Nutritional characterization and oxidative stability of α-linolenic acid in bread containing roasted ground flaxseed. LWT Food Sci. Technol. 2015, 61, 510–515. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Osuna, M.B.; Romero, C.A.; Romero, A.M.; Judis, M.A.; Bertola, N.C. Proximal composition, sensorial properties and effect of ascorbic acid and α-tocopherol on oxidative stability of bread made with whole flours and vegetable oils. LWT 2018, 98, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Feizollahi, E.; Hadian, Z.; Honarvar, Z. Food Fortification with Omega-3 Fatty Acids; Microencapsulation as an Addition Method. Curr. Nutr. Food Sci. 2017, 14, 90–103. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Strategies to extend bread and GF bread shelf-life: From Sourdough to antimicrobial active packaging and nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Giannone, V.; Lauro, M.R.; Spina, A.; Pasqualone, A.; Auditore, L.; Puglisi, I.; Puglisi, G. A novel α-amylase-lipase formulation as anti-staling agent in durum wheat bread. LWT Food Sci. Technol. 2016, 65, 381–389. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Polo, A.; Rizzello, C.G. The sourdough fermentation is the powerful process to exploit the potential of legumes, pseudo-cereals and milling by-products in baking industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 2158–2173. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Manini, F.; Brasca, M.; Plumed-Ferrer, C.; Morandi, S.; Erba, D.; Casiraghi, M.C. Study of the chemical changes and evolution of microbiota during sourdoughlike fermentation of wheat bran. Cereal Chem. 2014, 91, 342–349. [Google Scholar] [CrossRef]
- Neysens, P.; De Vuyst, L. Kinetics and modelling of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 95–103. [Google Scholar] [CrossRef]






| Sample Code | Formulation |
|---|---|
| Sourdough biga | Water 33%; sourdough 11%, strong wheat flour 56% |
| SB0 | Water 32%; sourdough biga16%, weak wheat flour 52% |
| SB5 | Water 32%; sourdough biga 16%, weak wheat flour 47%, flaxseed cake flour 5% |
| SB7.5 | Water 32%; sourdough biga 16%, weak wheat flour 44.5%, flaxseed cake flour 7.5% |
| SB10 | Water 32%; sourdough biga 16%, weak wheat flour 42%, flaxseed cake flour 10% |
| Baker’s Yeast biga | Water 31%; baker’s yeast 1%, strong wheat flour 68% |
| YB0 | Water 32%; baker’s yeast biga 16%, weak wheat flour 52% |
| YB5 | Water 32%; baker’s yeast biga 16%, weak wheat flour 47%, flaxseed cake flour 5% |
| YB7.5 | Water 32%; baker’s yeast biga 16%, weak wheat flour 44.5%, flaxseed cake flour 7.5% |
| YB10 | Water 32%; baker’s yeast biga 16%, weak wheat flour 42%, flaxseed cake flour 10% |
| p-Value 1 | Sourdough Biga | Baker’s Yeast Biga | |
|---|---|---|---|
| Dry matter (% dm) | * | 52.90 | 59.50 |
| pH | ** | 3.85 | 5.06 |
| Total titratable acidity (meq lactic acid/g dm) | *** | 0.12 | 0.02 |
| Acetic acid (mmol/g dm) | * | 0.06 | 0.02 |
| D-Lactic acid (mmol/g dm) | * | 0.03 | n.d. 2 |
| L-Lactic acid (mmol/g dm) | * | 0.06 | n.d. |
| Ethanol (mmol/g dm) | *** | 0.02 | 0.33 |
| p-Value 1 | SEM | SB0 | SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| water activity (aw) | n.s./n.s./n.s. | 0.01 | 0.96 | 0.96 | 0.96 | 0.96 | 0.95 | 0.97 | 0.96 | 0.96 |
| % of dry matter (% dm) | n.s./n.s./n.s. | 0.29 | 56.90 | 55.70 | 55.40 | 57.90 | 56.80 | 56.10 | 55.50 | 56.10 |
| free acidity (acidity degrees) | ***/§§§/¶¶¶ | 0.80 | 9.12c | 11.20b | 12.30b | 14.31a | 3.82 e | 4.72 d,e | 4.80 d,e | 5.19 d |
| palmitic acid (C16:0) | **/§/¶ | 0.92 | 15.22b | 11.05c | 15.26b | 11.84c | 18.25 a | 17.13 a | 15.14 b | 13.54 b |
| elaidic acid (C18:1t9) | ***/§§§/¶¶¶ | 0.19 | 0.48b | 0.57b | 0.82b | 1.28a | 0.13 c | 0.58 b | 0.79 b | 0.54 b |
| stearic acid (C18:0) | ***/§§§/¶¶¶ | 0.14 | 1.01b | 0.54c | 1.33a,b | 1.12b | 0.68 c | 1.04 b | 1.41 a | 1.56 a |
| oleic acid (C18:1c9) | n.s./§§§/¶¶ | 1.14 | 8.80c | 8.88c | 13.37b | 12.44b | 6.99 c | 9.82 c | 11.66 b,c | 21.02 a |
| linoleic acid (C18:2n-6) | n.s./§§§/¶ | 1.58 | 62.50a | 56.44b | 47.12c | 44.32c | 64.93 a | 56.22 b | 49.24 c | 37.00 d |
| linolenic acid (C18:3n-3) | ***/§§§/¶¶¶ | 0.53 | 3.67d | 19.07b | 17.35b | 23.98a | 3.76 d | 11.05 c | 17.14 b | 22.34 a |
| p-Value 1 | SEM | SB0 | SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid (mmol/g dm) | n.s/n.s/n.s. | 0.01 | 0.08 | 0.08 | 0.08 | 0.07 | 0.09 | 0.09 | 0.08 | 0.08 |
| D-Lactic acid (mmol/g dm) | ***/n.s./n.s. | 0.001 | 0.014 | 0.015 | 0.013 | 0.011 | n.d. | n.d. | n.d. | n.d. |
| L-Lactic acid (mmol/g dm) | ***/§/¶ | 0.005 | 0.040b | 0.055a | 0.054a | 0.050a | n.d. | n.d. | n.d. | n.d. |
| Ethanol (mmol/g dm) | ***/§§§/¶¶¶ | 0.007 | 0.050c | 0.050c | 0.060c | 0.065c | 0.066 c | 0.078 b | 0.116 a | 0.143 a |
| p-Value 1 | SEM | SB0 | SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | ***/§§§/¶¶¶ | 1.0 | 62.8b | 49.4c | 44.0e | 40.8f | 66.5 a | 46.8 d | 42.6 e,f | 43.4 e,f |
| a* | ***/§§§/¶¶¶ | 0.2 | −0.7e | 2.8d | 3.2c,d | 3.5b,c | −0.7 e | 3.2 c,d | 3.9 a,b | 4.1 a |
| b* | ***/§§§/¶¶¶ | 0.2 | 17.5a | 13.4b,c | 12.6c | 13.1b,c | 17.4 a | 14.0 b | 13.4 b,c | 13.4 b,c |
| SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 | |
|---|---|---|---|---|---|---|---|
| SB0 | 13.6 | 19.7 | 22.8 | 3.9 | 16.6 | 21.0 | 21.9 |
| SB5 | 5.4 | 9.3 | 16.7 | 3.9 | 8.1 | 9.0 | |
| SB7.5 | 3.3 | 23.3 | 3.3 | 1.8 | 4.7 | ||
| SB10 | 26.4 | 6.2 | 1.9 | 2.4 | |||
| YB0 | 20.3 | 24.6 | 25.5 | ||||
| YB5 | 4.3 | 4.9 | |||||
| YB7.5 | 5.5 |
| p-Value 1 | SEM | SB0 | SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total phenols (mg GAE/g dm) | */§§§/¶¶¶ | 0.073 | 0.481g | 0.671e | 0.932c | 1.041b | 0.301 h | 0.586 f | 0.833 d | 1.212 a |
| Total flavonoids (mg CAE/g dm) | */§§§/¶ | 0.039 | 0.083d | 0.165c | 0.216b | 0.241b | 0.075 d | 0.183 c | 0.226 b | 0.303 a |
| DPPH (μmol TE/g dm) | */§§§/n.s. | 0.186 | 0.505 | 1.734 | 2.329 | 2.826 | 0.361 | 1.657 | 2.283 | 2.710 |
| TEAC (μmoL TE/g dm) | n.s/§§§/¶ | 0.087 | 0.259d | 0.760c | 1.258b | 1.522a | 0.348 d | 0.606 c | 1.242 b | 1.545 a |
| SFA (g/100 g of fatty acids) | **/§§§/¶ | 0.81 | 20.46a | 17.43b | 17.74b | 13.66d | 20.01 a | 18.74 a | 18.58 a | 16.06 c |
| MUFA (g/100 g of fatty acids) | n.s./§§§/¶¶ | 1.00 | 10.57c | 10.40c | 15.26b | 16.41b | 8.43 d | 11.76 c | 13.61 b,c | 23.24 a |
| PUFA n-6 (g/100 g of fatty acids) | n.s./§§§/n.s. | 1.54 | 63.32 | 57.63 | 48.27 | 45.23 | 67.03 | 57.26 | 50.07 | 37.73 |
| PUFA n-3 (g/100 g of fatty acids) | ***/§§§/¶¶¶ | 0.50 | 3.79d | 19.18b | 18.12b | 23.98a | 3.80 d | 11.33 c | 17.21 b | 22.34 a |
| PUFA/SFA | n.s./n.s./n.s. | 0.56 | 3.28 | 4.41 | 3.74 | 5.07 | 3.54 | 3.66 | 3.62 | 3.74 |
| n-6/n-3 | n.s./§§§/n.s. | 0.48 | 16.81 | 3.01 | 2.66 | 1.89 | 17.64 | 5.05 | 2.94 | 1.69 |
| Parameter Evaluated by Panelist | p-Value 1 | SB0 | SB5 | SB7.5 | SB10 | YB0 | YB5 | YB7.5 | YB10 |
|---|---|---|---|---|---|---|---|---|---|
| Crumb | |||||||||
| Crumb color intensity (White scale) | *** | 5.85 a | 0.00 b | 0.00 b | 0.00b | 6.12 a | 0.00 b | 0.00 b | 0.00 b |
| Crumb color intensity (Brown scale) | *** | 0.00 d | 5.23 c | 6.55 a,b,c | 6.83 a,b | 0.00 d | 5.92 b,c | 7.38 a | 7.88 a |
| Alveoles dimension | *** | 6.63 a | 1.28 c | 1.10 c | 1.05 c | 2.95 b | 0.62 c | 0.74 c | 1.40 c |
| Homogeneity of alveolation | *** | 2.1 b | 7.30 a | 6.08 a | 8.53 a | 6.00 a | 8.32 a | 8.05 a | 7.08 a |
| Smell intensity | ** | 5.48 b | 8.03 a | 7.28 a | 7.13 a,b | 5.43 b | 6.45 a,b | 7.47 a | 6.70 a,b |
| Wheat smell | *** | 5.87 a | 0.62 b | 2.57 b | 1.33 b | 5.98 a | 3.22 a,b | 2.48 b | 3.40 a,b |
| Yeast smell | *** | 2.03 b | 0.47 b | 2.10 b | 1.13 b | 4.65 a | 2.75 a,b | 2.42 a,b | 2.58 a,b |
| Acetic smell | *** | 3.40 b | 2.27 b,c | 2.87 b,c | 6.30 a | 0.17 c | 1.75 b,c | 2.12 b,c | 1.25 b,c |
| Frankness | ** | 8.72 a | 7.97 a,b | 7.28 a,b | 5.85 a,b | 8.05 a,b | 7.53 a,b | 6.55 a,b | 4.78 b |
| Salted taste | *** | 3.17 a,b | 4.52 a | 2.98 a,b | 3.23 a,b | 1.98 b,c | 0.72 c | 0.78 c | 2.48 b,c |
| Acid taste | *** | 3.95 a,b | 5.10 a | 2.85 b | 4.83 a | 0.37 c | 0.82 c | 0.80 c | 0.95 c |
| Bitter taste | *** | 0.23 d | 1.12 c,d | 2.67 b,c | 5.30 a | 0.10 d | 0.53 c,d | 1.68 b,c,d | 3.65 a,b |
| Aftertaste | ** | 0.00 b | 0.23 a,b | 1.37 a,b | 1.63 a,b | 0.22 b | 0.20 b | 0.12 b | 2.08 a |
| Springiness | n.s. | 7.53 | 6.25 | 6.63 | 6.28 | 7.00 | 6.87 | 7.03 | 7.05 |
| Humidity of surface | n.s. | 3.67 | 4.27 | 5.05 | 4.28 | 3.63 | 4.37 | 4.33 | 4.53 |
| Crumb residual | n.s. | 1.43 | 1.30 | 2.58 | 1.70 | 2.58 | 2.85 | 2.32 | 2.55 |
| Resistance to chewing | *** | 6.80 a | 5.58 a,b | 3.68 b,c | 1.88 c | 4.30 b | 3.92 b,c | 3.98 b,c | 4.73 a,b |
| Juiciness | n.s. | 2.17 | 3.53 | 2.70 | 2.95 | 3.25 | 3.77 | 2.82 | 3.25 |
| Adhesiveness | ** | 5.83 a | 4.90 a,b | 3.80 a,b,c | 2.45 c | 3.68 a,b,c | 3.50 a,b,c | 3.40 b,c | 3.70 a,b,c |
| Crust | |||||||||
| Crispiness | *** | 4.90 d | 6.37 a,b,c,d | 7.02 a,b,c | 5.95 a,b,c,d | 5.55 c,d | 7.32 a,b | 7.63 a | 5.70 b,c,d |
| Hardness | *** | 3.10 b,c | 4.00 a,b,c | 4.95 a,b,c | 2.88 b,c | 2.77 c | 4.88 a,b,c | 5.33 a,b | 6.10 a |
| Smell intensity | *** | 4.34 a,b | 6.05 a | 5.53 a,b | 3.03 b | 4.07 a,b | 5.80 a | 6.57 a | 3.10 b |
| Salted taste | ** | 2.00 b | 3.13 a,b | 4.20 a | 3.60 a,b | 1.80 b | 2.43 a,b | 2.47 a,b | 2.45 a,b |
| Toasted taste | * | 1.98 b | 4.48 a,b | 5.30 a | 4.73 a,b | 4.82 a,b | 4.38 a,b | 4.92 a,b | 5.10 a |
| Bitter taste | *** | 0.12 c | 0.57 b,c | 2.80 a,b | 5.05 a | 0.55 b,c | 0.40 b,c | 0.92 b,c | 2.78 a,b |
| Aftertaste | *** | 0.00 b | 0.00 b | 1.02 a,b | 2.00 a | 0.00 b | 0.07 b | 0.03 b | 1.23 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Appl. Sci. 2020, 10, 5235. https://doi.org/10.3390/app10155235
Taglieri I, Sanmartin C, Venturi F, Macaluso M, Zinnai A, Tavarini S, Serra A, Conte G, Flamini G, Angelini LG. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Applied Sciences. 2020; 10(15):5235. https://doi.org/10.3390/app10155235
Chicago/Turabian StyleTaglieri, Isabella, Chiara Sanmartin, Francesca Venturi, Monica Macaluso, Angela Zinnai, Silvia Tavarini, Andrea Serra, Giuseppe Conte, Guido Flamini, and Luciana G. Angelini. 2020. "Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake" Applied Sciences 10, no. 15: 5235. https://doi.org/10.3390/app10155235