Natal Origin and Spatiotemporal Distribution of Leatherback Turtle (Dermochelys coriacea) Strandings at a Foraging Hotspot in Temperate Waters of the Southwest Atlantic Ocean
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
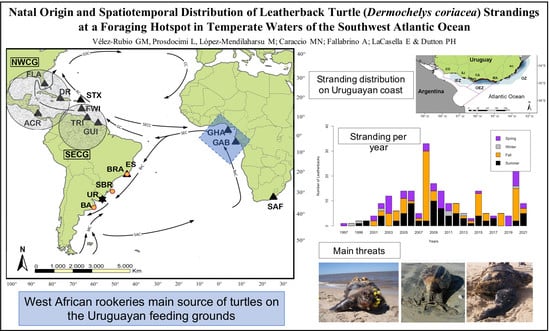
2.1. Study Area
2.2. Sample Collection
2.3. Laboratory Analysis
2.4. Sexual Maturity, State of Decomposition, and Cause of Stranding Determination
2.5. Data Analysis
3. Results
3.1. Genetic Diversity in Foraging Grounds
3.2. Size Distribution, Sex, and State of Decomposition
3.3. Spatiotemporal Distribution of the Strandings
3.4. Cause of Strandings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pritchard, P.C.H. International Migrations of South American Sea Turtles (Cheloniidae and Dermochelidae). Anim. Behav. 1973, 21, 18–27. [Google Scholar] [CrossRef]
- James, M.C.; Davenport, J.; Hays, G.C. Expanded Thermal Niche for a Diving Vertebrate: A Leatherback Turtle Diving into near-Freezing Water. J. Exp. Mar. Biol. Ecol. 2006, 335, 221–226. [Google Scholar] [CrossRef]
- López-Mendilaharsu, M.; Rocha, C.F.D.; Miller, P.; Domingo, A.; Prosdocimi, L. Insights on Leatherback Turtle Movements and High Use Areas in the Southwest Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 2009, 378, 31–39. [Google Scholar] [CrossRef]
- Marine Turtle Specialist Group-IUCN. Available online: https://www.iucn-mtsg.org (accessed on 10 August 2022).
- Wallace, B.P.; DiMatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Alberto Abreu-Grobois, F.; Amorocho, D.; Bjorndal, K.A.; et al. Regional Management Units for Marine Turtles: A Novel Framework for Prioritizing Conservation and Research across Multiple Scales. PLoS ONE 2010, 5, e15465. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Wallace, B.P.; Girondot, M. Leatherback Turtle (Dermochelys coriacea) Southwest Atlantic Ocean Subpopulation. The IUCN Red List of Threatened Species 2013. Available online: http://www.iucnredlist.org/details/46967817/0 (accessed on 12 July 2022).
- Vargas, S.M.; Barcelos, A.C.; Rocha, R.G.; Guimarães, P.; Amorim, L.; Martinelli, A.; Santos, F.R.; Erickson, J.; Marcondes, A.C.J.; Ludwig, S. Genetic Monitoring of the Critically Endangered Leatherback Turtle (Dermochelys coriacea) in the South West Atlantic. Reg. Stud. Mar. Sci. 2022, 55, 102530. [Google Scholar] [CrossRef]
- Komoroske, L.M.; Jensen, M.P.; Stewart, K.R.; Shamblin, B.M.; Dutton, P.H. Advances in the Application of Genetics in Marine Turtle Biology and Conservation. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Sales, G.; Giffoni, B.B.; Barata, P.C.R. Incidental Catch of Sea Turtles by the Brazilian Pelagic Longline Fishery. J. Mar. Biol. Assoc. UK 2008, 88, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Mrosovsky, N.; Ryan, G.D.; James, M.C. Leatherback Turtles: The Menace of Plastic. Mar. Pollut. Bull. 2009, 58, 287–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González Carman, V.; Alvarez, K.C.; Prosdocimi, L.; Inchaurraga, M.C.; Dellacasa, R.F.; Faiella, A.; Echenique, C.; Gonzalez, R.; Andrejuk, J.; Mianzan, H.W.; et al. Argentinian Coastal Waters: A Temperate Habitat for Three Species of Threatened Sea Turtles. Mar. Biol. Res. 2011, 7, 500–508. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Mortimer, J.A.; Seminoff, J.A.; Amorocho, D.; Bjorndal, K.A.; et al. Global Conservation Priorities for Marine Turtles. PLoS ONE 2011, 6, e24510. [Google Scholar] [CrossRef]
- Wallace, B.P.; Kot, C.Y.; Dimatteo, A.D.; Lee, T.; Crowder, L.B.; Lewison, R.L. Impacts of Fisheries Bycatch on Marine Turtle Populations Worldwide: Toward Conservation and Research Priorities. Ecosphere 2013, 4, 1–49. [Google Scholar] [CrossRef]
- Fossette, S.; Witt, M.J.; Miller, P.; Nalovic, M.A.; Albareda, D.; Almeida, A.P.; Broderick, A.C.; Chacón-Chaverri, D.; Coyne, M.S.; Domingo, A.; et al. Pan-Atlantic Analysis of the Overlap of a Highly Migratory Species, the Leatherback Turtle, with Pelagic Longline Fisheries. Proc. R. Soc. Lond. B Biol. Sci. 2014, 281, 20133065. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.; Eckert, S.A.; Bruno, S.C.; Scalfoni, J.T.; Giffoni, B.; López-Mendilaharsu, M.; Thomé, J.C.A. Satellite-Tracked Movements of Female Dermochelys coriacea from Southeastern Brazil. Endanger. Species Res. 2011, 15, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, V.R.F.; Mitraud, S.F.; Ferraz, M.L.C.P.; Lima, E.H.S.M.; Melo, M.T.D.; Santos, A.J.B.; da Silva, A.C.C.D.; de Castilhos, J.C.; Batista, J.A.F.; Lopez, G.G.; et al. Adaptive Threat Management Framework: Integrating People and Turtles. Environ. Dev. Sustain. 2016, 18, 1541–1558. [Google Scholar] [CrossRef]
- Thomé, J.C.A.; Baptisotte, C.; Moreira, L.M.; Scalfoni, J.T.; Almeida, A.P.; Rieth, D.B.; Barata, P.C.R. Nesting Biology and Conservation of the Leatherback Sea Turtle (Dermochelys coriacea) in the State of Espírito Santo, Brazil, 1988–1989 to 2003–2004. Chelonian. Conserv. Biol. 2007, 6, 15–27. [Google Scholar] [CrossRef]
- Soto, J.M.R.; Beheregaray, R.C.P.; Rebello, R.A.R.P.; De, P. Range Extension: Nesting by Dermochelys and Caretta in Southern Brazil. Mar. Turtle Newslett. 1997, 77, 6–7. [Google Scholar]
- Barata, P.C.R.; Fabiano, F.F.C. Evidence for Leatherback Sea Turtle (Dermochelys coriacea) Nesting in Arraial Do Cabo, State of Rio de Janeiro, and a Review of Occasional Leatherback Nests in Brazil. Mar. Turtle Newslett. 2011, 96, 13–16. [Google Scholar]
- Loebmann, D.; Legat, J.F.A.; Puchnick-Legat, A.; Camargo, R.C.R.; Erthal, S.; Severo, M.; De Góes, J.M. Dermochelys coriacea (Leatherback Sea Turtle) Nesting. Herpetol. Rev. 2008, 39, 81. [Google Scholar]
- Marcovaldi, M.A.; Thomé, J.; Fallabrino, A. (Eds.) Sea Turtles in the Atlantic Southwest Region. Draft Report to the IUCN-SSC Marine Turtle Specialist Group. 2021. Available online: https://www.iucn-mtsg.org/s/MTSG-RR_2021_SW-Atlantic_draft.pdf (accessed on 12 July 2022).
- Houghton, J.D.R.; Doyle, T.K.; Wilson, M.W.; Davenport, J.; Hays, G. Jellyfish Aggregations and Leatherback Turtle Foraging Patterns in a Temperate Coastal Environment. Ecology 2006, 87, 1967–1972. [Google Scholar] [CrossRef]
- James, M.C.; Ottensmeyer, C.A.; Myers, R.A. Identification of High-Use Habitat and Threats to Leatherback Sea Turtles in Northern Waters: New Directions for Conservation. Ecol. Lett. 2005, 8, 195–201. [Google Scholar] [CrossRef]
- Benson, S.R.; Eguchi, T.; Foley, D.G.; Forney, K.A.; Bailey, H.; Hitipeuw, C.; Samber, B.P.; Tapilatu, R.F.; Rei, V.; Ramohia, P.; et al. Large-Scale Movements and High-Use Areas of Western Pacific Leatherback Turtles. Dermochelys Coriacea. Ecosphere 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Evans, D.R.; Valverde, R.A.; Ordoñez, C.; Carthy, R.R. Identification of the Gulf of Mexico as an Important High-Use Habitat for Leatherback Turtles from Central America. Ecosphere 2021, 12, e03722. [Google Scholar] [CrossRef]
- Hays, G.C.; Houghton, J.D.R.; Isaacs, C.; King, R.S.; Lloyd, C.; Lovell, P. First Records of Oceanic Dive Profiles for Leatherback Turtles, Dermochelys coriacea, Indicate Behavioural Plasticity Associated with Long-Distance Migration. Anim. Behav. 2004, 67, 733–743. [Google Scholar] [CrossRef]
- Sale, A.; Luschi, P.; Mencacci, R.; Lambardi, P.; Hughes, G.R.; Hays, G.C.; Benvenuti, S.; Papi, F. Long-Term Monitoring of Leatherback Turtle Diving Behaviour during Oceanic Movements. J. Exp. Mar. Biol. Ecol. 2006, 328, 197–210. [Google Scholar] [CrossRef]
- Luschi, P.; Mencacci, R.; Hays, G.C. A Review of Migratory Behaviour of Sea Turtles off Southeastern Africa A Review of Patterns of Multiple Paternity Across Sea Turtle Rookeries View Project Innovative Methods to Track Sea Turtles View Project. S. Afr. J. Sci. 2006, 102, 51–58. [Google Scholar]
- Lambardi, P.; Lutjeharms, J.R.E.; Mencacci, R.; Hays, G.C.; Luschi, P. Influence of Ocean Currents on Long-Distance Movement of Leatherback Sea Turtles in the Southwest Indian Ocean. Mar. Ecol. Prog. Ser. 2008, 353, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Aleksa, K.T.; Sasso, C.R.; Nero, R.W.; Evans, D.R. Movements of Leatherback Turtles (Dermochelys coriacea) in the Gulf of Mexico. Mar. Biol. 2018, 165, 158. [Google Scholar] [CrossRef]
- Hays, G.C.; Houghton, J.D.R.; Myers, A.E. Pan-Atlantic Leatherback Turtle Movements. Nature 2004, 429, 522. [Google Scholar] [CrossRef]
- Frazier, J. Las Tortugas Marinas En El Atlántico Sur Occidental. Asoc. Herpetol. Argent. 1984, 2, 22. [Google Scholar]
- Vargas, S.M.; Araújo, F.C.F.; Monteiro, D.S.; Estima, S.C.; Almeida, A.P.; Soares, L.S.; Santos, F.R. Genetic Diversity and Origin of Leatherback Turtles (Dermochelys coriacea) from the Brazilian Coast. J. Hered. 2008, 99, 215–220. [Google Scholar] [CrossRef]
- Vargas, S.M.; Lins, L.S.F.; Molfetti, É.; Ho, S.Y.W.; Monteiro, D.; Barreto, J.; Colman, L.; Vila-Verde, L.; Baptistotte, C.; Thomé, J.C.A.; et al. Revisiting the Genetic Diversity and Population Structure of the Critically Endangered Leatherback Turtles in the South-West Atlantic Ocean: Insights for Species Conservation. J. Mar. Biol. Assoc. UK 2019, 99, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Prosdocimi, L.; Dutton, P.H.; Albareda, D.; Remis, M.I. Origin and Genetic Diversity of Leatherbacks (Dermochelys coriacea) at Argentine Foraging Grounds. J. Exp. Mar. Biol. Ecol. 2014, 458, 13–19. [Google Scholar] [CrossRef]
- Fossette, S.; Girard, C.; López-Mendilaharsu, M.; Miller, P.; Domingo, A.; Evans, D.; Kelle, L.; Plot, V.; Prosdocimi, L.; Verhage, S.; et al. Atlantic Leatherback Migratory Paths and Temporary Residence Areas. PLoS ONE 2010, 5, e13908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, M.J.; Augowet Bonguno, E.; Broderick, A.C.; Coyne, M.S.; Formia, A.; Gibudi, A.; Mounguengui Mounguengui, G.A.; Moussounda, C.; NSafou, M.; Nougessono, S.; et al. Tracking Leatherback Turtles from the World’s Largest Rookery: Assessing Threats across the South Atlantic. Proc. R Soc. Lond B Biol. Sci. 2011, 278, 2338–2347. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Mendilaharsu, M.; Sales, G.; Coluchi, R.; Marcovaldi, M.; Giffoni, B. At-Sea Distribution of Juvenile Leatherback Turtles: New Insights from Bycatch Data in the Atlantic Ocean. Mar. Ecol. Prog. Ser. 2019, 621, 199–208. [Google Scholar] [CrossRef]
- Laporta, M.; Miller, P.; Domingo, A. Captura Incidental de Tortugas Marinas En La Pesquería de Arrastre Uruguaya. In Proceedings of the Marine Turtles of the North East Atlantic: Contributions for the First Regional Conference, San Sebastian, Spain, 14–15 November 2008; Zaldua-Mendizabal, N., Egaña-Callejo, A., Eds.; Aranzadi Society of Sciences: San Sebastian, Spain, 2012; pp. 43–50. [Google Scholar]
- López-Mendilaharsu, M.; Sales, G.; Giffoni, B.; Miller, P.; Niemeyer Fiedler, F.; Domingo, A. Distribución y Composición de Tallas de Las Tortugas Marinas (Carretta caretta y Dermochelys coriacea) Que Interactúan Con El Palangre Pelagico En El Atlántico Sur. Col. Vol. Sci. Pap. ICCAT 2007, 60, 2094–2109. [Google Scholar]
- Vélez-Rubio, G.M.; Estrades, A.; Fallabrino, A.; Tomás, J. Marine Turtle Threats in Uruguayan Waters: Insights from 12 Years of Stranding Data. Mar. Biol. 2013, 160, 2797–2811. [Google Scholar] [CrossRef]
- Monteiro, D.S.; Estima, S.C.; Gandra, T.B.R.; Silva, A.P.; Bugoni, L.; Swimmer, Y.; Seminoff, J.A.; Secchi, E.R. Long-Term Spatial and Temporal Patterns of Sea Turtle Strandings in Southern Brazil. Mar. Biol. 2016, 163, 1–19. [Google Scholar] [CrossRef]
- Prosdocimi, L.; Teryda, N.S.; Navarro, G.S.; Carthy, R.R. Use of Remote Sensing Tools to Predict Focal Areas for Sea Turtle Conservation in the South-Western Atlantic. Aquat. Conserv. 2021, 31, 830–840. [Google Scholar] [CrossRef]
- Laporta, M.; Miller, P.; Horta, S.; Riestra, G. First Report of Leatherback Turtle Entanglement in Trap Lines in the Uruguayan Continental Shelf. Mar. Turt. Newls. 2006, 112, 9–11. [Google Scholar]
- Carreira, S.; Maneyro, R. Lista Roja de Los Anfibios y Reptiles Del Uruguay: Una Evaluación Del Estado de Conservación de La Herpetofauna de Uruguay; Dirección Nacional de Medio Ambiente; International Union for Conservation of Nature: Montevideo, Uruguay, 2015; ISBN 9789974658202. [Google Scholar]
- Vélez-Rubio, G.M.; Estrades, A.; Fallabrino, A.; Carreira, S. Libro Rojo de Los Anfibios y Reptiles Del Uruguay. Biología y conservación de los Anfibios y Reptiles en peligro de extinción a nivel nacional; Carreira, S., Maneyro, R., Eds.; DINAMA: Montevideo, Uruguay, 2019. [Google Scholar]
- Wildermann, N.; Gredzens, C.; Avens, L.; Barrios-Garrido, H.; Bell, I.; Blumenthal, J.; Bolten, A.; Braun McNeill, J.; Casale, P.; Di Domenico, M.; et al. Informing Research Priorities for Immature Sea Turtles through Expert Elicitation. Endanger. Species Res. 2018, 37, 55–76. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.A.E. Oceanografia Física. In Os Ecossistemas Costeiro e Marinho do Extremo Sul do Brazil; Seeliger, U., Ode-Brecht, C., Castello, J.P., Eds.; Ecoscientia: Rio Grande, Brazil, 1998; pp. 104–106. [Google Scholar]
- Ortega, L.; Martínez, A. Multiannual and Seasonal Variability of Water Masses and Fronts Over the Uruguayan Shelf. J. Coast. Res. 2007, 233, 618–629. [Google Scholar] [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Guerrero, R.A.; Favero, M.; Bava, J. Marine Fronts at the Continental Shelves of Austral South America: Physical and Ecological Processes. J. Mar. Syst. 2004, 44, 83–105. [Google Scholar] [CrossRef]
- Dutton, P.H. Methods for Collection and Preservation of Samples for Sea Turtle Genetic Studies. In Proceedings of the International Symposium on Sea Turtle Conservation Genetics; Bowen, B.W., Witzell, W.N., Eds.; NOAA National Marine Fisheries Service: Silver Spring, MD, USA, 1996; pp. 17–24. [Google Scholar]
- Eckert, K.L. Research and Management Techniques for the Conservation of Sea Turtles; IUCN/SSC Marine Turtle Specialist Group: Washington, DC, USA, 1999; p. 235. [Google Scholar]
- LaCasella, E.L.; Epperly, S.P.; Jensen, M.P.; Stokes, L.; Dutton, P.H. Genetic Stock Composition of Loggerhead Turtles Caretta caretta Bycaught in the Pelagic Waters of the North Atlantic. Endanger. Species Res. 2014, 22, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Dutton, P.H.; Roden, S.; Stewart, K.R.; La Casella, E.; Tiwari, M.; Formia, A.; Thome, J.; Livingstone, S.R.; Eckert, S.; Chacon-Chaverri, D.; et al. Population Stock Structure of Leatherback Turtles (Dermochelys coriacea) in the Atlantic Revealed Using MtDNA and Microsatellite Markers. Conserv. Genet. 2013, 14, 625–636. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Heled, J.; Kearse, M.; Moir, R.; Stones-Havas, S.; Sturrock, S.; et al. Geneious v 6. 2011. Available online: http://www.geneious.com/ (accessed on 12 July 2022).
- Lahanas, P.N.; Bjorndal, K.A.; Bolten, A.B.; Encalada, S.E.; Miyamoto, M.M.; Valverde, R.A.; Bowen, B.W. Genetic Composition of a Green Turtle (Chelonia mydas) Feeding Ground Population: Evidence for Multiple Origins. Mar. Biol. 1998, 130, 345–352. [Google Scholar] [CrossRef]
- Naro-Maciel, E.; Becker, J.H.; Lima, E.H.S.M.; Marcovaldi, M.Â.; DeSalle, R. Testing Dispersal Hypotheses in Foraging Green Sea Turtles (Chelonia mydas) of Brazil. J. Hered. 2007, 98, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Caraccio, M.N. Análisis de La Composición Genética de Chelonia mydas (Tortuga verde) En El Área de Alimentación y Desarrollo de Uruguay. Master’s Thesis, Universidad de la República, Montevideo, Uruguay, 2008. [Google Scholar]
- Caraccio, M.N.; Domingo, A.; Márquez, A.; Naro-Maciel, E.; Miller, P.; Pereira, A. Las Aguas Del Atlántico Sudoccidental y Su Importancia En El Ciclo de Vida de La Tortuga Cabezona (Caretta caretta): Evidencias a Través Del Anélisis Del ADNmt. Col. Vol. Sci. Pap. ICCAT 2008, 62, 1831–1837. [Google Scholar]
- Monzó Arguello, C.; Ló Pez-Jurado, L.F.; Rico, C.; Marco, A.; Ló Pez, P.; Hays, G.C.; Lee, P.L.M.; Lee, P.L.M. Evidence from Genetic and Lagrangian Drifter Data for Transatlantic Transport of Small Juvenile Green Turtles. J. Biogeogr. 2010, 37, 1752–1766. [Google Scholar] [CrossRef]
- Prosdocimi, L.; González Carman, V.; Albareda, D.A.; Remis, M.I. Genetic Composition of Green Turtle Feeding Grounds in Coastal Waters of Argentina Based on Mitochondrial DNA. J. Exp. Mar. Biol. Ecol. 2012, 412, 37–45. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; ISBN 9780231886710. [Google Scholar] [CrossRef] [Green Version]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin Version 3.0, an Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Pella, J.J.; Masuda, M. Bayesian Methods for Analysis of Stock Mixtures from Genetic Characters. Fish. Bull. NOAA 2001, 9, 151–167. [Google Scholar]
- Carreras, C.; Godley, B.J.; León, Y.M.; Hawkes, L.A.; Revuelta, O.; Raga, J.A.; Tomás, J. Contextualising the Last Survivors: Population Structure of Marine Turtles in the Dominican Republic. PLoS ONE 2013, 8, e66037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molfetti, É.; Torres Vilaça, S.; Georges, J.-Y.; Plot, V.; Delcroix, E.; Le Scao, R.; Lavergne, A.; Barrioz, S.; dos Santos, F.R.; de Thoisy, B. Recent Demographic History and Present Fine-Scale Structure in the Northwest Atlantic Leatherback (Dermochelys coriacea) Turtle Population. PLoS ONE 2013, 8, e58061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritz, C. Defining ‘evolutionarily significant units’ for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-162; 2023. Available online: https://CRAN.R-project.org/package=nlme (accessed on 12 July 2022).
- RStudio Team RStudio: Integrated Development Environment for R; RS Team: Boston, MA, USA, 2022.
- ESRI ArcGIS Desktop Help: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2011.
- Wallace, B.P.; Posnik, Z.A.; Hurley, B.J.; DiMatteo, A.D.; Bandimere, A.; Rodriguez, I.; Maxwell, S.M.; Meyer, L.; Brenner, H.; Jensen, M.P.; et al. Marine Turtle Regional Management Units 2.0: An Updated Framework for Conservation and Research of Wide-Ranging Megafauna Species. Endanger. Species Res. in press.
- Dutton, P.H.; Hitipeuw, C.; Zein, M.; Benson, S.R.; Petro, G.; Pita, J.; Rei, V.; Ambio, L.; Bakarbessy, J. Status and Genetic Structure of Nesting Populations of Leatherback Turtles (Dermochelys coriacea) in the Western Pacific. Chelonian. Conserv. Biol. 2007, 6, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Shamblin, B.M.; Bolten, A.B.; Abreu-Grobois, F.A.; Bjorndal, K.A.; Cardona, L.; Carreras, C.; Clusa, M.; Monzón-Argüello, C.; Nairn, C.J.; Nielsen, J.T.; et al. Geographic Patterns of Genetic Variation in a Broadly Distributed Marine Vertebrate: New Insights into Loggerhead Turtle Stock Structure from Expanded Mitochondrial DNA Sequences. PLoS ONE 2014, 9, e85956. [Google Scholar] [CrossRef] [Green Version]
- Wongfu, C.; Prasitwiset, W.; Poommouang, A.; Buddhachat, K.; Brown, J.L.; Chomdej, S.; Kampuansai, J.; Kaewmong, P.; Kittiwattanawong, K.; Nganvongpanit, K. Genetic Diversity in Leatherback Turtles (Dermochelys coriacea) along the Andaman Sea of Thailand. Diversity 2022, 14, 764. [Google Scholar] [CrossRef]
- Stewart, K.R.; LaCasella, E.L.; Roden, S.E.; Jensen, M.P.; Stokes, L.W.; Epperly, S.P.; Dutton, P.H. Nesting Population Origins of Leatherback Turtles Caught as Bycatch in the U.S. Pelagic Longline Fishery. Ecosphere 2016, 7, e01272. [Google Scholar] [CrossRef] [Green Version]
- Farrell, J.A.; Whitmore, L.; Mashkour, N.; Rollinson Ramia, D.R.; Thomas, R.S.; Eastman, C.B.; Burkhalter, B.; Yetsko, K.; Mott, C.; Wood, L.; et al. Detection and Population Genomics of Sea Turtle Species via Noninvasive Environmental DNA Analysis of Nesting Beach Sand Tracks and Oceanic Water. Mol. Ecol. Resour. 2022, 22, 2471–2493. [Google Scholar] [CrossRef] [PubMed]
- Colman, L.P. Ecology and Conservation of the Leatherback Sea Turtle (Dermochelys coriacea) Nesting in Brazil. Ph.D. Thesis, University of Exeter, Exeter, UK, 2018. [Google Scholar]
- Stewart, K.; Johnson, C.; Godfrey, M.H. The Minimum Size of Leatherbacks at Reproductive Maturity, with a Review of Sizes for Nesting Females from the Indian, Atlantic and Pacific Ocean Basins. Herpetol. J. 2007, 17, 123–128. [Google Scholar]
- Hays, G.C.; Hobson, V.J.; Metcalfe, J.D.; Righton, D.; Sims, D.W. Flexible Foraging Movements of Leatherback Turtles across the North Atlantic Ocean. Ecology 2006, 87, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Benson, S.R.; Dutton, P.H.; Réveillère, A.; Jacob, G.; Meetoo, C.; Dehecq, A.; Fossette, S. Oceanic Dispersal of Juvenile Leatherback Turtles: Going beyond Passive Drift Modeling. Mar. Ecol. Prog. Ser. 2012, 457, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Lalire, M. A Model for Simulating the Active Dispersal of Juvenile Sea Turtles with a Case Study on Western Pacific Leatherback Turtles. PLoS ONE 2017, 12, e0181595. [Google Scholar] [CrossRef] [Green Version]
- Estrades, A.; López-Mendilaharsu, M.; Fallabrino, A. Dermochelys coriacea (Leatherback Sea Turtle) Diet. Herpetol. Rev. 2007, 38, 330. [Google Scholar]
- Nagata, R.M.; Haddad, M.A.; Nogueira, M. The Nuisance of Medusae (Cnidaria, Medusozoa) to Shrimp Trawls in Central Part of Southern Brazilian Bight, from the Perspective of Artisanal Fishermen. Pan-Am. J. Aquat. Sci. 2009, 4, 312–325. [Google Scholar]
- Schiariti, A.; Dutto, M.S.; Pereyra, D.Y.; Siquier, G.F.; Morandini, A.C. Medusae (Scyphozoa and Cubozoa) from Southwestern Atlantic and Subantarctic Region (32-60°s, 34-70°W): Species Composition, Spatial Distribution and Life History Traits. Lat. Am. J. Aquat. Res. 2018, 46, 240–257. [Google Scholar] [CrossRef]
- Alvarez Colombo, G.; Mianzan, H.; Madirolas, A. Acoustic Characterization of Gelatinous-Plankton Aggregations: Four Case Studies from the Argentine Continental Shelf. ICES J. Mar. Sci. 2003, 60, 650–657. [Google Scholar] [CrossRef]
- Cabreira, A.G.; Madirolas, A.; Alvarez Colombo, G.; Acha, E.M.; Mianzan, H.W. Acoustic Study of the Río de La Plata Estuarine Front. ICES J. Mar. Sci. 2006, 63, 1718–1725. [Google Scholar] [CrossRef] [Green Version]
- Piola, A.R. The Influence of the Plata River Discharge on the Western South Atlantic Shelf. Geophys. Res. Lett. 2005, 32, L01603. [Google Scholar] [CrossRef]
- Ciotti, Á.M.; Odebrecht, C.; Fillmann, G.; Moller, O.O. Freshwater Outflow and Subtropical Convergence Influence on Phytoplankton Biomass on the Southern Brazilian Continental Shelf. Cont. Shelf. Res. 1995, 15, 1737–1756. [Google Scholar] [CrossRef]
- Möller, O.O.; Piola, A.R.; Freitas, A.C.; Campos, E.J.D. The Effects of River Discharge and Seasonal Winds on the Shelf off Southeastern South America. Cont. Shelf. Res. 2008, 28, 1607–1624. [Google Scholar] [CrossRef]
- Franco, B.C.; Defeo, O.; Piola, A.R.; Barreiro, M.; Yang, H.; Ortega, L.; Gianelli, I.; Castello, J.P.; Vera, C.; Buratti, C.; et al. Climate Change Impacts on the Atmospheric Circulation, Ocean, and Fisheries in the Southwest South Atlantic Ocean: A Review. Clim. Chang. 2020, 162, 2359–2377. [Google Scholar] [CrossRef]
- Nagaoka, S.M.; Ferro De Godoy, D.; Boussamba, F.L.; Formia, A.; Sounguet, G.-P. Unusual Mortality Event of Leatherback Turtles (Dermochelys coriacea) in the Southern Coast of Soo Paulo State, Brazil. Mar. Turtle Newslett. 2019, 156, 21–25. [Google Scholar]
- Rojas-Cañizales, D.; Espinoza-Rodríguez, N.; Rodríguez, M.A.; Palmar, J.; Montiel-Villalobos, M.G.; Wildermann, N.E.; Barrios-Garrido, H. Leatherback Turtles (Dermochelys coriacea) in the Gulf of Venezuela: An Update Stranding Assessment 2001–2014. Mar. Fish Sci. 2021, 34, 113–119. [Google Scholar] [CrossRef]
- Peltier, H.; Dabin, W.; Daniel, P.; Van Canneyt, O.; Dorémus, G.; Huon, M.; Ridoux, V. The Significance of Stranding Data as Indicators of Cetacean Populations at Sea: Modelling the Drift of Cetacean Carcasses. Ecol. Indic. 2012, 18, 278–290. [Google Scholar] [CrossRef]
- Hart, K.M.; Mooreside, P.; Crowder, L.B. Interpreting the Spatio-Temporal Patterns of Sea Turtle Strandings: Going with the Flow. Biol. Conserv. 2006, 129, 283–290. [Google Scholar] [CrossRef]
- Cantor, M.; Barreto, A.S.; Taufer, R.M.; Giffoni, B.; Castilho, P.V.; Maranho, A.; Beatriz, C.; Kolesnikovas, C.; Godoy, D.; Rogério, D.W.; et al. High Incidence of Sea Turtle Stranding in the Southwestern Atlantic Ocean. ICES J. Mar. Sci. 2020, 77, 1864–1878. [Google Scholar] [CrossRef]
- Marín, Y.H.; Defeo, O.; Horta, S. So Far and so Close: Opportunities for Marine Spatial Planning in the Southwest Atlantic Ocean. Ocean. Coast Manag. 2021, 211, 105737. [Google Scholar] [CrossRef]
- Marín, Y.; Guzmán, L.; Chocca, J.F.; Gómez, M. Zonas de Fondeo, Interferencias Con Actividades Pesqueras Uruguayas, y Elementos Para La Planificación Espacial Marina En El Río de La Plata. Rev. Transp. Territ. 2018, 19, 221–238. [Google Scholar] [CrossRef]
- Prosdocimi, L.; Albareda, D.A.; Bruno, I.; Rodriguez-Heredia, S. Navarro G Movimientos Estacionales de La Tortuga Laúd (Dermochelys coriacea) y Su Posible Interacción Con La Pesquería de Arrastre Costero En El Río de La Plata. Frente Marítimo 2016, 24, 147–154. [Google Scholar]
- Domingo, A.; Bugoni, L.; Prosdocimi, L.; Miller, P.; Laporta, M.; Monteiro, D.S.; Estrades, A.; Albareda, D. The Impact Generated on Sea Turtles by Fisheries in the Southwestern Atlantic Ocean; WWF Programa Marino para Latinoamérica y el Caribe: San José, Costa Rica, 2006. [Google Scholar]
- González Carman, V.; Acha, E.M.; Maxwell, S.M.; Albareda, D.; Campagna, C.; Mianzan, H. Young Green Turtles, Chelonia mydas, Exposed to Plastic in a Frontal Area of the SW Atlantic. Mar. Pollut. Bull. 2014, 78, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mianzan, H.; Pájaro, M.; Alvarez Colombo, G.; Madirolas, A. Feeding on Survival-Food: Gelatinous Plankton as a Source of Food for Anchovies. Hydrobiologia 2001, 451, 45–53. [Google Scholar] [CrossRef]
- Eastman, C.B.; Farrell, J.A.; Whitmore, L.; Rollinson Ramia, D.R.; Thomas, R.S.; Prine, J.; Eastman, S.F.; Osborne, T.Z.; Martindale, M.Q.; Duffy, D.J. Plastic Ingestion in Post-Hatchling Sea Turtles: Assessing a Major Threat in Florida Near Shore Waters. Front. Mar. Sci. 2020, 7, 693. [Google Scholar] [CrossRef]






| Nesting Areas | Foraging Areas | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NWCG | SECG | ||||||||||||||
| Haplotype | BRA | ACR | FLA | DR | STX | TRI | GUI | FWI | GHA | GAB | SAF | ES | SBR | BA | UR |
| Dc1.1 | 20 | 119 | 209 | 38 | 98 | 65 | 115 | 23 | 47 | 178 | 34 | 4 | 63 | 26 | 43 |
| Dc1.3 | 11 | 12 | 8 | 4 | 8 | ||||||||||
| Dc1.4 | 1 | 7 | 2 | 1 | |||||||||||
| Dc1.7 | 1 | ||||||||||||||
| Dc2.1 | 21 | ||||||||||||||
| Dc3.1 | 15 | 10 | 10 | 4 | 4 | 11 | 28 | 5 | 5 | 3 | 2 | 2 | |||
| Dc3.2 | 2 | 11 | 26 | 1 | |||||||||||
| Dc4.1 | 1 | 2 | 1 | 3 | |||||||||||
| Dc9.1 | 2 | 1 | |||||||||||||
| Dc13.1 | 1 | 1 | 35 | 14 | 2 | 4 | |||||||||
| Dc17.1 | 3 | ||||||||||||||
| Dc19.1 | 1 | ||||||||||||||
| DcA5 | 6 | ||||||||||||||
| DcC3 | 2 | ||||||||||||||
| N | 36 | 132 | 222 | 42 | 123 | 87 | 177 | 29 | 61 | 232 | 41 | 8 | 94 | 33 | 59 |
| h | 0.531 | 0.183 | 0.112 | 0.1765 | 0.338 | 0.415 | 0.533 | 0.352 | 0.379 | 0.387 | 0.298 | 0.678 | 0.524 | 0.371 | 0.425 |
| NFpy | 50 | 2500 | 75 | 35 | 250 | 3000 | 5000 | 55 | 100 | 5000 | 50 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Rubio, G.M.; Prosdocimi, L.; López-Mendilaharsu, M.; Caraccio, M.N.; Fallabrino, A.; LaCasella, E.L.; Dutton, P.H. Natal Origin and Spatiotemporal Distribution of Leatherback Turtle (Dermochelys coriacea) Strandings at a Foraging Hotspot in Temperate Waters of the Southwest Atlantic Ocean. Animals 2023, 13, 1285. https://doi.org/10.3390/ani13081285
Vélez-Rubio GM, Prosdocimi L, López-Mendilaharsu M, Caraccio MN, Fallabrino A, LaCasella EL, Dutton PH. Natal Origin and Spatiotemporal Distribution of Leatherback Turtle (Dermochelys coriacea) Strandings at a Foraging Hotspot in Temperate Waters of the Southwest Atlantic Ocean. Animals. 2023; 13(8):1285. https://doi.org/10.3390/ani13081285
Chicago/Turabian StyleVélez-Rubio, Gabriela M., Laura Prosdocimi, Milagros López-Mendilaharsu, Maria Noel Caraccio, Alejandro Fallabrino, Erin L. LaCasella, and Peter H. Dutton. 2023. "Natal Origin and Spatiotemporal Distribution of Leatherback Turtle (Dermochelys coriacea) Strandings at a Foraging Hotspot in Temperate Waters of the Southwest Atlantic Ocean" Animals 13, no. 8: 1285. https://doi.org/10.3390/ani13081285