Environmental and Anthropogenic Influences on Coliform Concentrations in the Octopus insularis Production Chain in the Veracruz Reef System, Gulf of Mexico
Abstract
:Simple Summary
Abstract
1. Introduction
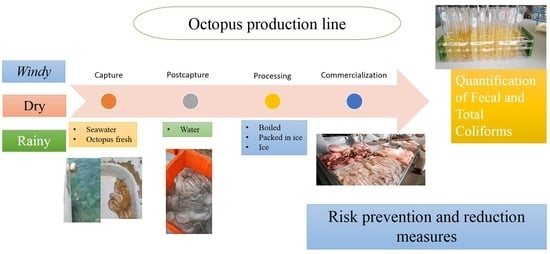
2. Materials and Methods
2.1. Sampling Strategy

2.2. Determination of Fecal and Total Coliforms in Sea Water, Fresh Water, Ice and Octopuses
2.3. Statistical Analysis
2.4. Environmental Parameters
3. Results
3.1. Total Coliforms by Stage of Octopus Production Chain
3.2. Fecal Coliforms by Stage of Octopus Production Chain
3.3. Environmental Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA). Manual de Buenas Prácticas de Manufactura para Productos Acuícolas y Pesqueros; Dirección Regional de Inocuidad de los Alimentos: San Salvador, El Salvador, 2017; pp. 18–24. [Google Scholar]
- FAO; World Health Organization. Code of Practice for Fish and Fishery Products; FAO: Rome, Italy, 2020; p. 372. [Google Scholar]
- Gutiérrez-Ruiz, C.; Román-Vives, M.; Vergara, C.; Badano, E. Impact of anthropogenic disturbances on the diversity of shallow stony corals in the Veracruz Reef System National Park. Rev. Mex. Biodivers. 2011, 82, 249–260. [Google Scholar] [CrossRef]
- Stark, J.S.; Bridgen, P.; Dunshea, G.; Galton-Fenzi, B.; Hunter, J.; Johnstone, G.; King, C.; Leeming, R.; Palmer, A.; Smith, J.; et al. Dispersal and dilution of wastewater from an ocean outfall at Davis Station, Antarctica, and resulting environmental contamination. Chemosphere 2016, 152, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Pena-Puch, A.; Pérez-Jiménez, J.C.; Espinoza-Tenorio, A. Advances in the study of Mexican fisheries with the social-ecological system (SES) perspective and its inclusion in fishery management policy. Ocean. Coast. Manag. 2019, 185, 105065. [Google Scholar] [CrossRef]
- Estrella-Gómez, N.; Escalante-Réndiz, D.; González-Borgos, A.; Sosa-Cordero, D.; Rojas-Herrera, R. Análisis microbiológico del pulpo rojo en puertos pesqueros de Campeche, Mexico. Salud Pública Mex. 2016, 58, 453–460. [Google Scholar] [CrossRef] [PubMed]
- CONAPESCA. Anuario Estadístico de Acuacultura y Pesca 2017; Comisión Nacional de Acuacultura y Pesca: Ciudad de Mexico, Mexico, 2017. [Google Scholar]
- Galindo-Cortés, G.; Hernández-Flores, A.; Santos-Valencia, J. Pulpo del Golfo de Mexico Octopus maya y Octopus vulgaris. In Sustentabilidad y Pesca Responsable en Mexico, Evaluación y Manejo; Beléndez-Moreno, L.F., Espino-Barr, E., Galindo-Cortes, G., Gaspar-Dillanes, M.T., Huidobro-Campos, L., Morales-Bojórquez, E., Eds.; Instituto Nacional de Pesca: Ciudad de Mexico, Mexico, 2014; pp. 179–210. [Google Scholar]
- Jiménez-Badillo, M.L.; Castro-Gaspar, L. Pesca artesanal en el Parque Nacional Sistema Arrecifal Veracruzano, Mexico. In Investigaciones Científicas en el Sistema Arrecifal Veracruzano; Granados Barba, A., Abarca Arenas, L.G., Vargas-Hernández, J.M., Eds.; Universidad Autónoma de Campeche: Ciudad de Mexico, Mexico, 2007; pp. 221–240. [Google Scholar]
- Jiménez-Badillo, M.L. Manejo de la pesquería de pulpo en el estado de Veracruz con énfasis en el Sistema Arrecifal Veracruzano. In Manejo de los Recursos Pesqueros de la Cuenca del Golfo de Mexico y del Mar Caribe; Aldana, A.D., Enriquez, D.M., Elías, V., Eds.; Colección La Ciencia en Veracruz; Editorial de la Universidad Veracruzana: Veracruz, Mexico, 2013; Volume 1, pp. 229–236. [Google Scholar]
- Pardo, F. Diseño de una Estrategia de Evaluación de Cadenas Productivas para el Desarrollo Sustentable de Comunidades Pesqueras Ribereñas. Estudio de Caso: Antón Lizardo, Municipio Alvarado, Veracruz, Mexico. Master’s Thesis, Instituto de Investigaciones y Estudios Superiores de las Ciencias Administrativas, Universidad Veracruzana: Veracruz, Mexico, 2007; p. 128. [Google Scholar]
- SAGARPA. Acuerdo Por el Que se da a Conocer la Actualización de la Carta Nacional Pesquera; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2018; 11/06/2018. [Google Scholar]
- Jiménez-Badillo, M.L. Geographic information system: A tool to manage the octopus fishery in the Veracruz Reef System National Park, Mexico. In GIS/Spatial Analyses in Fishery and Aquatic Sciences; Nishida, T., Caton, A.E., Eds.; Fishery GIS Society: Saitama, Japan, 2010; Volume 4, pp. 319–328. [Google Scholar]
- SAGARPA. Acuerdo por el que se da a Conocer el Plan de Manejo Pesquero de Pulpo (O. maya y O. vulgaris) del Golfo de Mexico y Mar Caribe; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2014; 28/03/2014. [Google Scholar]
- CONAPESCA. Anuario Estadístico de Acuacultura y Pesca 2020; Comisión Nacional de Acuacultura y Pesca: Ciudad de Mexico, Mexico, 2020. [Google Scholar]
- Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA). Manual de Introducción a la Inocuidad de los Alimentos; Dirección Regional de Inocuidad de los Alimentos: San Salvador, El Salvador, 2018. [Google Scholar]
- Contreras, D.; Vázquez, A.; Romero, Y.; Pardo, J.; Guevara, M.; Rivera, J. Plan Estratégico: Plataforma Tecnológica Pulpo Maya Para el Desarrollo de Productos de Alto Valor Agregado; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C (CIATEJ): Jalisco, Mexico, 2019. [Google Scholar]
- Robert, M. Microorganismos indicadores de la calidad del agua potable en Cuba. Rev. CENIC Cienc. Biol. 2014, 45, 32–43. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Secretaría de Salud. NOM-251-SSA1-2009, Prácticas de Higiene para el Proceso de Alimentos, Bebidas o Suplementos Alimenticios; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2008; 10/10/2008. [Google Scholar]
- Schlitzer, R. Ocean Data View. 2023. Available online: http://odv.awi.de (accessed on 5 September 2023).
- Food and Drug Administration. Bacteriological Analytical Manual, 9th ed; AOAC International: Arlington, TX, USA, 2004; Chapter 4. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Secretaría de Salud. NOM-112-SSA1-1994, Bienes y Servicios. Determinación de Bacterias Coliformes. Técnica del Número Más Probable; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 1995; 10/19/1995. [Google Scholar]
- Secretaría de Salud. NOM-201-SSA1-2015, Productos y Servicios. Agua y Hielo para Consumo Humano, Envasados y a Granel. Especificaciones Sanitarias y Métodos de Prueba; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2015; 12/22/2015. [Google Scholar]
- Ortíz-Lozano, L.; Pérez-España, H.; Granados-Barba, A.; González-Gándara, C.; Gutiérrez-Velázquez, A.; Martos, J. The Reef Corridor of the Southwest Gulf of Mexico: Challenges for its management and conservation. Ocean. Coast. Manag. 2013, 86, 22–32. [Google Scholar] [CrossRef]
- APIVER. Datos Estadísticos del Movimiento de Carga Enero-Junio. 2019. Available online: http://www.puertodeveracruz.com.mx/wp-content/uploads/2019/07/Estadistica_Apiver_junio_2019 (accessed on 13 February 2022).
- Ortíz-Lozano, L. Identification of priority conservation actions in marine protected areas: Using a causal networks approach. Ocean. Coast. Manag. 2012, 55, 74–83. [Google Scholar] [CrossRef]
- SEMARNAT. Informe de la Situación del Medio Ambiente en Mexico. Compendio de Estadísticas Ambientales. Indicadores Clave, de Desempeño Ambiental y de Crecimiento Verde; SEMARNAT: Ciudad de Mexico, Mexico, 2016. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.; Beher, J.; Jones, K.; Possingham, H.; Wood, P.; Fekete, B.; Levy, M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Owens, W.; Tchounwou, P. The impact of rainfall on fecal coliform bacteria in Bayou Dorcheat (North Louisiana). Int. J. Envirom Res. Health 2006, 3, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Mijin Seo, L.; Lee, H.; Yongseok, K. Relationship between Coliform Bacteria and Water Quality Factors at Weir Stations in the Nakdong River, South Korea. Water 2019, 11, 1171. [Google Scholar] [CrossRef]
- Thériault, A.; Duchesne, S. Quantifying the fecal coliform loads in urban watersheds by hydrologic/hydraulic modeling: Case study of the Beuport river watershed in Quebec. Water 2015, 7, 615–633. [Google Scholar] [CrossRef]
- Jiménez-Badillo, M.L.; Cruz-Rodas, S.; Lozano-Aburto, M.A.; Rodríguez-Quiroz, G. Problemática ambiental y socioeconómica del Parque Nacional Sistema Arrecifal Veracruzano. Investig. Cienc. 2014, 60, 58–64. [Google Scholar]
- Secretaría de Salud. NOM-242-SSA1-2009, Productos y Servicios. Productos de la Pesca Frescos, Refrigerados, Congelados y Procesados. Especificaciones Sanitarias y Métodos de Prueba; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2011. [Google Scholar]
- Cho, K.; Pachepsky, Y.; Oliver, D.; Muirhead, R.; Park, Y.; Quilliam, R.; Shelton, D. Modeling fate and transport of fecally-derived at the watershed scale: State of the science and future opportunities. Water Res. 2016, 100, 38–56. [Google Scholar] [CrossRef] [PubMed]
- SAGARPA-SENASICA. Manual de Buenas Prácticas de Manejo a Bordo en Embarcaciones Menores; SAGARPA: Ciudad de Mexico, Mexico, 2015. [Google Scholar]
- Steffen, R.; Hill, D.; DuPont, H. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Salas-Pérez, J.; Salas-Monreal, D.; Monreal-Gómez, M.; Riverón-Enzástiga, M.; Llasat, C. Seasonal absolute acustic intensity, atmospheric forcing and currents in a tropical coral reef system. Estuarine. Coast. Shelf Sci. 2012, 100, 102–112. [Google Scholar] [CrossRef]
- Shawyer, M.; Medina, A. El Uso del Hielo en Pequeñas Embarcaciones de Pesca; Documento Técnico de Pesca; FAO: Roma, Italy, 2005; p. 436. [Google Scholar]



| Stages | Zone | Climatic Seasons | Sample | TC (MPN ± σ) | N | p |
|---|---|---|---|---|---|---|
| Capture | Southern zone (Enmedio) | Dry | Sea water | 14.4 ± 13.9 a | 6 | 0.2305 NS |
| Rainy | Sea water | 1.8 ± 0 a | 6 | |||
| Windy | Sea water | 8.15 ± 8.19 a | 12 | |||
| Dry | Fresh octopus | 2.4 ± 1.04 a | 3 | 0.0273 * | ||
| Rainy | Fresh octopus | 22 ± 1.73 b | 3 | |||
| Windy | Fresh octopus | 91.1 ± 45.92 c | 6 | |||
| Southern zone (Chopa) | Dry | Sea water | 3.15 ± 1.48 a | 6 | 0.0608 NS | |
| Rainy | Sea water | 1.8 ± 0 a | 6 | |||
| Windy | Sea water | 25.15 ± 25.47 a | 12 | |||
| Dry | Fresh octopus | 3.73 ± 3.00 a | 3 | 0.0141 * | ||
| Rainy | Fresh octopus | 37.33 ± 7.51 b | 3 | |||
| Windy | Fresh octopus | 286.83 ± 447.55 c | 3 | |||
| Northern zone (La Gallega) | Dry | Sea water | 1.8 ± 0 a | 6 | 1 NS | |
| Rainy | Sea water | 1.8 ± 0 a | 6 | |||
| Windy | Sea water | 1.8 ± 0 a | 6 | |||
| Dry | Fresh octopus | 61.33 ± 12.22 a | 3 | 0.0273 * | ||
| Rainy | Fresh octopus | 1373.33 ± 392.59 b | 3 | |||
| Windy | Fresh octopus | 34.73 ±8.81 c | 3 | |||
| Between zones (Southern, Northern) | Fresh octopus | 0.4202 NS | ||||
| Post-capture | Southern zone | Dry | Freshwater | 540 ± 0 a | 3 | 0.0273 * |
| Rainy | Freshwater | 240 ± 0 b | 3 | |||
| Windy | Freshwater | 79 ± 0 c | 3 | |||
| Northern zone | Not applicable | Not applicable | ||||
| Processing | Both zones | Not applicable | Boiled octopus | 226.06 ± 290.82 a | 9 | 0.3063 NS |
| Fresh octopus | 212.53 ± 457.61 a | 36 | ||||
| Commercialization | Both zones | Not applicable | Boiled octopus | 226.06 ± 290.82 a | 9 | 0.003 * |
| Octopus packed in ice | 853.93 ± 463.87 b | 9 | ||||
| Ice | 1600 ± 0 | 9 |
| Stages | Zone | Climatic Seasons | Sample | FC (MPN ± σ) | N | p |
|---|---|---|---|---|---|---|
| Capture | Southern zone (Enmedio) | Dry | Sea water | 4.5 ± 0 a | 3 | 0.3827 NS |
| Rainy | Sea water | 0 a | 3 | |||
| Windy | Sea water | 1.8 ± 0 a | 3 | |||
| Dry | Fresh octopus | 0 a | 3 | 0.0495 * | ||
| Rainy | Fresh octopus | 6.7 ± 1.91 b | 3 | |||
| Windy | Fresh octopus | 4.7 ± 0 c | 3 | |||
| Southern zone (Chopa) | Dry | Sea water | 0 | 6 | Not applicable | |
| Rainy | Sea water | 0 | 6 | |||
| Windy | Sea water | 0 | 6 | |||
| Dry | Fresh octopus | 1.87 ± 0.12 a | 3 | 0.0201 * | ||
| Rainy | Fresh octopus | 29.67 ±5.77 b | 3 | |||
| Windy | Fresh octopus | 1.3 ± 0.52 c | 3 | |||
| Northern zone (La Gallega) | Dry | Sea water | 0 | 6 | Not applicable | |
| Rainy | Sea water | 0 | 6 | |||
| Windy | Sea water | 0 | 6 | |||
| Dry | Fresh octopus | 56.33 ± 20.03 a | 3 | 0.0273 * | ||
| Rainy | Fresh octopus | 203.33 ± 63 b | 3 | |||
| Windy | Fresh octopus | 14.5 ± 8.89 c | 6 | |||
| Between zones (Southern, Northern) | Fresh octopus | 0.0129 * | ||||
| Post-capture | Southern zone | Dry | Freshwater | 7.8 ± 0 a | 3 | 0.0273 * |
| Rainy | Freshwater | 33 ± 0 b | 3 | |||
| Windy | Freshwater | 12.4 ± 0 c | 3 | |||
| Northern zone | Not applicable | Not applicable | ||||
| Processing | Both zones | Not applicable | Boiled octopus | 18.91 ± 36.27 a | 12 | 0.6566 NS |
| Fresh octopus | 21.50 ± 46.65 a | 29 | ||||
| Commercialization | Both zones | Not applicable | Boiled octopus | 18.91 ± 36.27 a | 12 | |
| Octopus packed in ice | 266.72 ±339.76 b | 12 | 0.0041 * | |||
| Ice | 818.75 ± 816.03 c | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña-Ramírez, S.; Jiménez-Badillo, M.d.L.; Galindo-Cortes, G.; Marval-Rodríguez, A.; Castañeda-Chávez, M.d.R.; Reyes-Velázquez, C.; Rodulfo-Carvajal, H.; De Donato-Capote, M. Environmental and Anthropogenic Influences on Coliform Concentrations in the Octopus insularis Production Chain in the Veracruz Reef System, Gulf of Mexico. Animals 2023, 13, 3049. https://doi.org/10.3390/ani13193049
Acuña-Ramírez S, Jiménez-Badillo MdL, Galindo-Cortes G, Marval-Rodríguez A, Castañeda-Chávez MdR, Reyes-Velázquez C, Rodulfo-Carvajal H, De Donato-Capote M. Environmental and Anthropogenic Influences on Coliform Concentrations in the Octopus insularis Production Chain in the Veracruz Reef System, Gulf of Mexico. Animals. 2023; 13(19):3049. https://doi.org/10.3390/ani13193049
Chicago/Turabian StyleAcuña-Ramírez, Sarai, María de Lourdes Jiménez-Badillo, Gabriela Galindo-Cortes, Angel Marval-Rodríguez, María del Refugio Castañeda-Chávez, Christian Reyes-Velázquez, Hectorina Rodulfo-Carvajal, and Marcos De Donato-Capote. 2023. "Environmental and Anthropogenic Influences on Coliform Concentrations in the Octopus insularis Production Chain in the Veracruz Reef System, Gulf of Mexico" Animals 13, no. 19: 3049. https://doi.org/10.3390/ani13193049