Platelet- Rich Plasma Treatment Supported by Ultrasound Detection of Septa in Recurrent Canine Aural Hematoma: A Case Series
Abstract
:Simple Summary
Abstract
1. Introduction
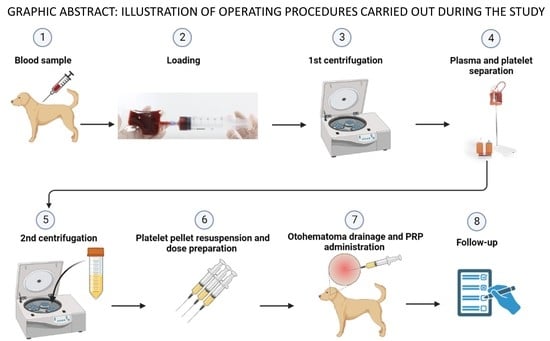
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Inclusion Criteria
2.3. Autologous Platelet- Rich Plasma Preparation
2.4. PRP Treatments
2.5. Clinical Evaluation
3. Results
3.1. Quality of PRP
3.2. Clinical Study Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pachaly, J.R.; Quessada, A.M.; Belettini, S.T.; Borges, T.B.; Sala, P.L.; Tramontin, R.S.; Souza, M.V.F.; Voltarelli-Pachaly, E.M. Treating Otohematomas in Dogs with Intra-Lesional Corticotherapy. Acta Sci. Vet. 2021, 49, 110065. [Google Scholar] [CrossRef]
- Hewitt, J.; Bajwa, J. Aural hematoma and its treatment: A review. Can. Vet. J. 2020, 61, 313–315. [Google Scholar] [PubMed]
- MacPhail, C. Current Treatment Options for Auricular Hematomas. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 635–641. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Lee, Y.H.; Brodbelt, D.C.; Church, D.B.; Pegram, C.; Halfacree, Z. Reporting the epidemiology of aural haematoma in dogs and proposing a novel aetiopathogenetic pathway. Sci. Rep. 2021, 11, 21670. [Google Scholar] [CrossRef]
- Dubielzig, R.R.; Wilson, J.W.; Seireg, A.A. Pathogenesis of canine aural haematoma. J. Am. Vet. Med. Assoc. 1984, 158, 873–875. [Google Scholar]
- Lund, E. Identifying aural hematoma risk. Banfield J. 2006, 2, 16–22. [Google Scholar]
- Bacon, N.J. Veterinary Surgery: Small Animal, 2nd ed.; Johnston, S.A., Tobias, K.M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; pp. 2309–2327. [Google Scholar]
- Pavletic, M.M. Use of laterally placed vacuum drains for management of aural hematomas in five dogs. J. Am. Vet. Med. Assoc. 2015, 247, 112–117. [Google Scholar] [CrossRef]
- Yool, D.A. Part IV-Head and Neck surgery, Chapter 19- Ear Surgery. Small Anim. Soft Tissue Surg. CABI 2012, SF911.Y66, 292–308. [Google Scholar]
- Romatowski, J. Nonsurgical treatment of aural hematomas. J. Am. Vet. Med. Assoc. 1994, 204, 1318. [Google Scholar]
- Hall, J.; Weir, S.; Ladlow, J. Treatment of canine aural haematoma by UK veterinarians. J. Small Anim. Pract. 2016, 57, 360–364. [Google Scholar] [CrossRef]
- Kuwahara, J. Canine and feline aural hematomas: Results of treatment with corticosteroids. J. Am. Anim. Hosp. Assoc. 1986, 22, 641–647. Available online: https://eurekamag.com/research/001/312/001312173.php (accessed on 16 June 2023).
- Cordero, A.M.; Marquez, C.L.; Nunez, C.R.; Cardenas, R.H.; Waisburd, G.S.; Ortega, A.F. Non-surgical treatment of canine auricular haematoma with intralesional and systemic corticosteroids, a pilot study. Vet. Sci. Med. 2020, 3, 1–4. Available online: https://sciaeon.org/articles/Non-surgical-Treatment-of-Canine-Auricular-Hematoma-with-Intralesional-and-Systemic-Corticosteroids-A-Pilot-Study.pdf (accessed on 16 June 2023).
- Hedlund, C. Section 3—Surgery of the head and neck. Chapter 21—Incisional Drainage of Aural Hematomas. In Complications in Small Animal Surgery; Griffon, D., Hamaide, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 150–154. [Google Scholar] [CrossRef]
- Lahiani, J.; Niebauer, G.W. On the nature of canine aural haematoma and its treatment with continuous vacuum drainage. J. Small Anim. Pract. 2020, 61, 195–201. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Leslie, M. Beyond clotting: The powers of platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-rich plasma peptides: Key for regeneration. Int. J. Pept. 2012, 2012, 532519. [Google Scholar] [CrossRef] [PubMed]
- Crovetti, G.; Martinelli, G.; Issi, M.; Barone, M.; Guizzardi, M.; Campanati, B.; Moroni, M.; Carabelli, A. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. 2004, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tambella, A.M.; Attili, A.R.; Dini, F.; Palumbo-Piccionello, A.; Vullo, C.; Serri, E.; Scrollavezza, P.; Dupré, G. Autologous platelet gel to treat chronic decubital ulcers: A randomized, blind controlled clinical trial in dogs. Vet. Surg. 2014, 43, 726–733. [Google Scholar] [CrossRef]
- Bosch, G.; van Schie, H.T.; De Groot, M.W.; Cadby, J.A.; van de Lest, C.H.A.; Barneveld, A.; van Weeren, P.R. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J. Orthopaed Res. 2009, 28, 211–217. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Iacopetti, I.; Patruno, M.; Melotti, L.; Martinello, T.; Bedin, S.; Badon, T.; Righetto, E.M.; Perazzi, A. Autologous Platelet-Rich Plasma Enhances the Healing of Large Cutaneous Wounds in Dogs. Front. Vet. Sci. 2020, 7, 575449. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Collins, T.; Alexander, D.; Barkatali, B. Platelet-rich plasma: A narrative review. EFORT Open Rev. 2021, 6, 225–235. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Yin, J. Platelet-Rich Plasma: A Promising Product for Treatment of Peripheral Nerve Regeneration After Nerve Injury. Neuroscience 2011, 121, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Borrione, P.; Gianfrancesco, A.; Pereira, M.T.; Pigozzi, F. Platelet-Rich Plasma in Muscle Healing. Am. J. Phys. Med. Rehab. 2010, 89, 854–861. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open. Sport. Exerc. Med. 2016, 4, e000060. [Google Scholar] [CrossRef]
- Franklin, S.P.; Garner, B.C.; Cook, J.L. Characteristics of canine platelet-rich plasma prepared with five commercially available systems. Am. J. Vet. Res. 2015, 76, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Canapp, S.O.; Mason, D.R.; Cox, C.; Hess, T. Canine Platelet-Rich Plasma Systems: A Prospective Analysis. Front. Vet. Sci. 2016, 2, 73. [Google Scholar] [CrossRef]
- Perego, R.; Spada, E.; Moneta, E.; Baggiani, L.; Proverbio, D. Use of Autologous Leucocyte- and Platelet-Rich Plasma (L-PRP) in the Treatment of Aural Hematoma in Dogs. Vet. Sci. 2021, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Day, M.J. Immunopathogenesis of canine aural haematoma. J. Small Anim. Pract. 1997, 38, 152–158. [Google Scholar] [CrossRef]
- Lanz, O.I.; Wood, B.C. Surgery of the ear and pinna. Vet. Clin. N. Am. Small Anim. Pract. 2004, 24, 567–599. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, J. Canine and feline aural hematoma: Clinical, experimental, and clinicopathologic observations. Am. J. Vet. Res. 1986, 47, 2300–2308. Available online: https://europepmc.org/article/med/3490809 (accessed on 16 June 2023).
- Eviatar, A. Tragal perichondrium and cartilage in reconstructive ear surgery. Laringoscope 1978, 88, 1–23. [Google Scholar] [CrossRef]
- Fahie, A.M.; Ortolano, G.A.; Guercio, V.; Schaffer, J.A.; Johnston, G.; Au, J.; Hettlich, B.A.; Phillips, T.; Allen, M.J.; Bertone, A.L. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2013, 243, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Textor, J. Autologous biologic treatment for equine musculoskeletal injuries: Platelet-rich plasma and IL-1 receptor antagonist protein. Vet Clin. N. Am. Equine Pract. 2011, 27, 275–298. [Google Scholar] [CrossRef]
- Attili, A.-R.; Iacoucci, C.; Serri, E.; Cuteri, V.; Cantalamessa, A.; Linardi, M.; Rifici, C.; Mazzullo, G.; Rossi, G.; Galosi, L.; et al. Antibacterial Properties of Canine Platelet-Rich Plasma and Other Non-Transfusional Hemo-Components: An in vitro Study. Front Vet Sci. 2021, 8, 746809. [Google Scholar] [CrossRef]
- Cieślik-Bielecka, A.; Bold, T.; Ziółkowski, G.; Pierchała, M.; Królikowska, A.; Reichert, P. Antibacterial Activity of Leukocyte- and Platelet-Rich Plasma: An In Vitro Study. BioMed Res. Int. 2018, 2018, 9471723. [Google Scholar] [CrossRef]
- Çetinkaya, R.; Yenilmez, E.; Petrone, P.; Yılmaz, S.; Bektöre, B.; Şimsek, B.; Atik, T.K.; Özyurt, M.; Ünlü, A. Platelet-rich plasma as an additional therapeutic option for infected wounds with multi-drug resistant bacteria: In vitro antibacterial activity study. Eur. J. Trauma Emerg. Surg. 2019, 45, 555–565. [Google Scholar] [CrossRef]
- Rossi, L.A.; Murray, I.R.; Chu, C.R.; Muschler, G.F.; Rodeo, S.A.; Piuzzi, N.S. Classification systems for platelet-rich plasma. Bone Jt. J. 2019, 101-B, 891–896. [Google Scholar] [CrossRef]
- De Long, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. Am. 2017, 99, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Benigni, L.; Lamb, C. Diagnostic imaging of ear disease in the dog and cat. Practice 2006, 28, 122–130. Available online: https://bvajournals.onlinelibrary.wiley.com/doi/pdf/10.1136/inpract.28.3.122 (accessed on 16 June 2023). [CrossRef]
- Perego, R.; Proverbio, D.; Baggiani, L.; Moneta, E.; Spada, E. Clinical efficacy of autologous platelet-rich plasma (PRP) in canine perianal fistulas and aural hematomas. J. Vet. Intern. Med. 2017, 31, 265–266, Speech Presented at the 26th ECVIM-CA Congress held in Gothenburg in 2016. Available online: https://air.unimi.it/handle/2434/782469 (accessed on 16 June 2023).


| Whole Blood Concentration | PRP Concentration ± SD | Concentration Factor | % Recovery | p Value | |
|---|---|---|---|---|---|
| WBC | 7.56 × 103/μL | 2.60 × 103/μL ± 1.28 × 103/μL | 0.34 | 3.5% | * |
| RBC | 6.12 × 106/μL | 4.98 × 105/μL ± 3.31 × 105/μL | 0.08 | 1.0% | * |
| PLT | 2.57 × 105/μL | 1.18 × 106/μL ± 6.07 × 105/μL | 5 | 51.5% | * |
| Dog | Type | Drained mL D0 | Previous Scars D0 | Scars Related to PRP Treatment | N° of Non- Communicating Chambers | N° of Communicating Chambers | PRP Infiltrations | mL of PRP | Bandage | Days for Healing | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CASE 1L | Acute | 12 | NO | NO | 0 | 1 | 2 | 2 | NO | 40 | 20 |
| CASE 1R | Acute | 20 | NO | NO | 0 | 2 | 1 | 2 | NO | 20 | 14 |
| CASE 2 | Acute | 20 | NO | NO | 0 | 1 | 1 | 2 | NO | 37 | 19 |
| CASE 3 | Acute | 14 | NO | NO | 0 | 1 | 1 | 2 | YES | 10 | 19 |
| CASE 4 | Chronic | 8 * | YES | NO | 0 | 1 | 1 | 2 | NO | 14 | 18 |
| CASE 5 | Acute | 25 | NO | NO | 0 | 1 | 2 | 2 | YES | 60 | 14 |
| CASE 6 | Acute | 35 | NO | NO | 0 | 1 | 1 | 2 | NO | 30 | 12 |
| CASE 7 | Acute | 18 | NO | NO | 0 | 1 | 1 | 2 | NO | 30 | 12 |
| CASE 8 | Acute | 10 | NO | NO | 0 | 1 | 2 | 2 | NO | 60 | 11 |
| CASE 9 | Acute | 10 | YES | NO | 0 | 1 | 2 | 2 | NO | 60 | 10 |
| CASE 10 | Chronic | 10 | YES | NO | 0 | 1 | 2 | 2 | YES | 90 | 10 |
| CASE 11 | Acute | 40 | NO | NO | 2 | 3 | 2 | 2 | YES | 11 | 16 |
| MEAN | 18.5 | 1.5 | 38.5 | 14.58 | |||||||
| ST.DEV. | 10.3 | 0.52 | 24.7 | 3.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palagiano, P.; Graziano, L.; Scarabello, W.; Berni, P.; Andreoli, V.; Grolli, S. Platelet- Rich Plasma Treatment Supported by Ultrasound Detection of Septa in Recurrent Canine Aural Hematoma: A Case Series. Animals 2023, 13, 2456. https://doi.org/10.3390/ani13152456
Palagiano P, Graziano L, Scarabello W, Berni P, Andreoli V, Grolli S. Platelet- Rich Plasma Treatment Supported by Ultrasound Detection of Septa in Recurrent Canine Aural Hematoma: A Case Series. Animals. 2023; 13(15):2456. https://doi.org/10.3390/ani13152456
Chicago/Turabian StylePalagiano, Paola, Lisa Graziano, Walter Scarabello, Priscilla Berni, Valentina Andreoli, and Stefano Grolli. 2023. "Platelet- Rich Plasma Treatment Supported by Ultrasound Detection of Septa in Recurrent Canine Aural Hematoma: A Case Series" Animals 13, no. 15: 2456. https://doi.org/10.3390/ani13152456