Impact of Cadmium and Lead Exposure on Camel Testicular Function: Environmental Contamination and Reproductive Health
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
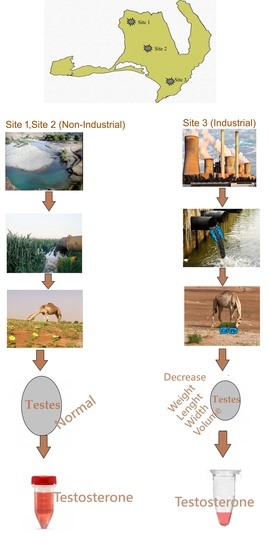
2.1. Experimental Design
2.2. Animals
2.3. Testicular Size and Volume
2.4. Hormonal Assay
2.5. Biochemical Analysis
2.6. Histologic Assessment
2.7. Assessment of Seminiferous Tubules and Maturation Using Histology
2.8. Statistical Analysis
3. Results
3.1. Quantification of Pb and Cd in Water
3.2. Quantification of Heavy Metals in Soil
3.3. Quantification of Heavy Metals in Plants
3.4. Quantification of Pb and Cd in Serum
3.5. Quantification of Heavy Metals in Testes
3.6. Quantification of Heavy Metals in Urine
3.7. Impact of Pb and Cd Exposure on the Testicular Dimension and Volume
3.8. Impact of Pb and Cd Exposure on Testicular Steroidogenesis
3.9. The Impact of Pb and Cd Exposure on the Histological Characteristics of the Testes
3.10. Seminiferous Tubule Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balali-Mood, M.; Riahi-Zanjani, B.; Yousefzadeh, H.; Sadeghi, M. Concentrations of mercury, lead, chromium, cadmium, arsenic and aluminum in irrigation water wells and wastewaters used for agriculture in Mashhad, northeastern Iran. Int. J. Occup. Environ. Med. 2013, 4, 80–86. [Google Scholar]
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. 2016, 21, 65. [Google Scholar] [PubMed]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef]
- Massadeh, A.M.; Snook, R.D. Determination of Pb and Cd in road dusts over the period in which Pb was removed from petrol in the UK. J. Environ. Monit. 2002, 4, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.; Arowojolu, A.O.; Shittu, O.B.; Anetor, J.I. Cadmium toxicity: A possible cause of male infertility in Nigeria. Reprod. Biol. 2006, 6, 17–30. [Google Scholar] [PubMed]
- Rosa, H.J.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Rosa, M.D.; Zarrilli, S.; Paesano, L.; Carbone, U.; Boggia, B.; Petretta, M.; Maisto, A.; Cimmino, F.; Puca, G.; Colao, A. Traffic pollutants affect fertility in men. Hum. Reprod. 2003, 18, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Formicki, G.; Stawarz, R.; Lukač, N.; Putała, A.; Kuczkowska, A. Combined effects of cadmium and ultraviolet radiation on mortality and mineral content in common frog (Rana temporaria) larvae. J. Environ. Sci. Health Part A 2008, 43, 1174–1183. [Google Scholar] [CrossRef]
- Yadav, R.; Goyal, B.; Sharma, R.; Dubey, S.; Minhas, P. Post-irrigation impact of domestic sewage effluent on composition of soils, crops and ground water—A case study. Environ. Int. 2002, 28, 481–486. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Wang, Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ. Int. 2005, 31, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, R. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Árvay, J.; Záhorcová, Z.; Tomáš, J.; Hauptvogl, M.; Stanovič, R.; Harangozo, Ľ. Mercury in edible wild-grown mushrooms from historical mining area–Slovakia: Bioaccumulation and risk assessment. J. Microbiol. Biotechnol. Food Sci. 2021, 4, 1–4. [Google Scholar] [CrossRef]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Kawatra, B.; Bakhetia, P. Consumption of heavy metal and minerals by adult women through food in sewage and tube-well irrigated area around Ludhiana city (Punjab, India). J. Hum. Ecol. 2008, 23, 351–354. [Google Scholar] [CrossRef]
- Alam, M.; Snow, E.; Tanaka, A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total Environ. 2003, 308, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Atlanta, G. Cadmium Toxicity–Case Studies in Environmental Medicine; The National Acadmic Press: Washington, DC, USA, 2012. [Google Scholar]
- Chirinos-Peinado, D.M.; Castro-Bedriñana, J.I. Lead and cadmium blood levels and transfer to milk in cattle reared in a mining area. Heliyon 2020, 6, e03579. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2013.
- Wong, C.-H.; Mruk, D.D.; Lui, W.-Y.; Cheng, C.Y. Regulation of blood-testis barrier dynamics: An in vivo study. J. Cell Sci. 2004, 117, 783–798. [Google Scholar] [CrossRef] [Green Version]
- Takiguchi, M.; Yoshihara, S.I. New aspects of cadmium as endocrine disruptor. Environ. Sci. 2006, 13, 107–116. [Google Scholar]
- Sadik, N.A. Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J. Biochem. Mol. Toxicol. 2008, 22, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.-T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. The apical ES–BTB–BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol. Med. 2013, 19, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.L.; Havstad, S.; Ownby, D.R.; Peterson, E.L.; Maliarik, M.; McCabe, M.J., Jr.; Barone, C.; Johnson, C.C. Blood lead level and risk of asthma. Environ. Health Perspect. 2005, 113, 900–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, D.E.; Wilson, J.; Dixon, S.L.; Smith, J.; Evens, A. The relationship of housing and population health: A 30-year retrospective analysis. Environ. Health Perspect. 2009, 117, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Kianoush, S.; Balali-Mood, M.; Mousavi, S.R.; Moradi, V.; Sadeghi, M.; Dadpour, B.; Rajabi, O.; Shakeri, M.T. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin. Pharmacol. Toxicol. 2012, 110, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Sarkar, S.; Patil, R.; Tripathi, H. Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: A biochemical and histopathological study in male rats. Arch. Environ. Contam. Toxicol. 2007, 53, 667–677. [Google Scholar] [CrossRef]
- Huang, H.; An, Y.; Jiao, W.; Wang, J.; Li, S.; Teng, X. CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ. Sci. Pollut. Res. 2018, 25, 18838–18845. [Google Scholar] [CrossRef]
- Leidens, D.; Bianchini, A.; Varela Junior, A.S.; Barcarolli, I.F.; Rosa, C.E.; Bonnel, J.; Calabuig, C.P.; Corcini, C.D. Effects of experimental lead exposure on testis of the Chestnut Capped Blackbird Chrysomus ruficapillus. Bull. Environ. Contam. Toxicol. 2018, 100, 324–330. [Google Scholar] [CrossRef]
- Sujatha, K.; Srilatha, C.; Anjaneyulu, Y.; Rao, T.; Sreenivasulu, D.; Amaravathi, P. Effect of lead acetate on sperm morphology and testis of wistar albino rats. Indian J. Anim. Reprod. 2011, 32, 63–67. [Google Scholar]
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004, 229, 383–392. [Google Scholar] [CrossRef]
- Chen, H.; Chen, K.; Zhao, F.; Guo, Y.; Liang, Y.; Wang, Z.; Liu, T.; Chen, S. Macroautophagy involved in testosterone synthesis in Leydig cells of male dairy goat (Capra hircus). Theriogenology 2022, 180, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lu, X.; Cen, X.; Chen, X.; Li, F.; Zhong, S. RNA-Seq identifies key reproductive gene expression alterations in response to cadmium exposure. BioMed. Res. Int. 2014, 2014, 529271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupertino, M.C.; Novaes, R.D.; Santos, E.C.; Neves, A.C.; Silva, E.; Oliveira, J.A.; Matta, S.L. Differential susceptibility of germ and leydig cells to cadmium-mediated toxicity: Impact on testis structure, adiponectin levels, and steroidogenesis. Oxidative Med. Cell. Longev. 2017, 2017, 3405089. [Google Scholar] [CrossRef]
- Aithamadouche, N.; Nesrine, S.; Kharoubi, O.; Slimani, M.; Aoues, A. The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Not. Sci. Biol. 2013, 5, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Biswas, N.; Ghosh, P. Effect of lead on male gonadal activity in albino rats. Kathmandu Univ. Med. J. (KUMJ) 2004, 2, 43–46. [Google Scholar]
- Bala, A.; Junaidu, A.U.; Salihu, M.D.; Agaie, B.M.; Saulawa, M.A.; Musawa, A.I.; Ahmad, K.H. Determination of heavy metal residues in slaughtered camels at sokoto and Gusau Modern Abattoirs, Nigeria. J. Health Pollut. 2018, 8, 181204. [Google Scholar] [CrossRef] [Green Version]
- Földi, J.; Kulcsar, M.; Pecsi, A.; Huyghe, B.; De Sa, C.; Lohuis, J.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Hill, A.; Patterson, K.Y.; Veillon, C.; Morris, E. Digestion of biological materials for mineral analyses using a combination of wet and dry ashing. Anal. Chem. 1986, 58, 2340–2342. [Google Scholar] [CrossRef]
- Ennab, W.; Mustafa, S.; Wei, Q.; Lv, Z.; Kavita, N.M.; Ullah, S.; Shi, F. Resveratrol protects against restraint stress effects on stomach and spleen in adult male mice. Animals 2019, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Ennab, W.; Ye, N.; Wu, H.; Ullah, S.; Hadi, T.; Bassey, A.P.; Mustafa, S.; Jiang, J.; Wei, Q.; Shi, F. The Synergistic Effects of the Combination of L-Carnitine and Lycopene on the Lycopene Bioavailability and Duodenal Health of Roosters. Animals 2023, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Ennab, W.; Nazar, K.; Wei, Q.; Lv, Z.; Shi, Z.; Shi, F. Positive roles of resveratrol in early development of testicular germ cells against maternal restraint stress in mice. Animals 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MX, K.N.; Jiang, J.; Enayatullah, H.; Ennab, W.; Mustafa, S.; Rodeni, S.; Wei, Q.; Shi, F. Sweet taste receptor agonists alter ovarian functions and ovarian cycles in aged mice. Reprod. Biol. 2019, 19, 230–236. [Google Scholar]
- Ullah, S.; Mustafa, S.; Ennab, W.; Muhammad, J.; Shafiq, M.; Kavita, N.M.; Lü, Z.-P.; Mao, D.-G.; Shi, F.-X. A protective role of resveratrol against the effects of immobilization stress in corpora lutea of mice in early pregnancy. J. Integr. Agric. 2020, 19, 1857–1866. [Google Scholar] [CrossRef]
- Oliveira Filho, A.B.; Souza, R.S.D.; Azeredo-Oliveira, M.T.V.D.; Peruquetti, R.L.; Cedenho, A.P. Microdissection testicular sperm extraction causes spermatogenic alterations in the contralateral testis. Genet. Mol. Res. 2010, 9, 1405–1413. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 104–108.
- GB 5749-2006; Standards for Drinking Water Quality. Ministry of Health of China; Standardization Administration of China: Beijing, China, 2006.
- Dart, R.C. Medical Toxicology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Luckey, T.D.; Venugopal, B. Metal toxicity in mammals. In Physiologic and Chemical Basis for Metal Toxicity; Plenum Press: New York, NY, USA, 1977; Volume 1. [Google Scholar]
- Tahar, K.; Keltoum, B. Effects of heavy metals pollution in soil and plant in the industrial area, West Algeria. J. Korean Chem. Soc. 2011, 55, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Pugh, R.E.; Dick, D.G.; Fredeen, A.L. Heavy metal (Pb, Zn, Cd, Fe, and Cu) contents of plant foliage near the Anvil Range lead/zinc mine, Faro, Yukon Territory. Ecotoxicol. Environ. Saf. 2002, 52, 273–279. [Google Scholar] [CrossRef]
- Karabasanavar, N.S.; Sivaraman, G.; Girish, P. Metal residues in retail chicken meat at Shivamogga, Karnataka. J. Meat Sci. 2020, 15, 75–79. [Google Scholar] [CrossRef]
- ALINORM 01/12A; Food Additives and Contaminants, Joint Codex Alimentarius Commission. FAO/WHO; Food standards Programme: Rome, Italy, 2001.
- Norouzirad, R.; González-Montaña, J.-R.; Martínez-Pastor, F.; Hosseini, H.; Shahrouzian, A.; Khabazkhoob, M.; Malayeri, F.A.; Bandani, H.M.; Paknejad, M.; Foroughi-Nia, B. Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci. Total Environ. 2018, 635, 308–314. [Google Scholar] [CrossRef]
- Ajarem, J.S.; Hegazy, A.K.; Allam, G.A.; Allam, A.A.; Maodaa, S.N.; Mahmoud, A.M. Heavy metal accumulation, tissue injury, oxidative stress, and inflammation in dromedary camels living near petroleum industry sites in Saudi Arabia. Animals 2022, 12, 707. [Google Scholar] [CrossRef]
- Damek-Poprawa, M.; Sawicka-Kapusta, K. Histopathological changes in the liver, kidneys, and testes of bank voles environmentally exposed to heavy metal emissions from the steelworks and zinc smelter in Poland. Environ. Res. 2004, 96, 72–78. [Google Scholar] [CrossRef]
- de Oliveira, C.P.A.; Carneiro, A.A.; Ervilha, L.O.G.; Machado-Neves, M.; Souza, A.C.F.; Carvalho, R.P.R. Does environmental pollution affect male reproductive system in naturally exposed vertebrates? A systematic review. Theriogenology 2023, 198, 305–316. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]
- Rajendar, B.; Bharavi, K.; Rao, G.; Kishore, P.; Kumar, P.R.; Kumar, C.; Kumar, D.S. Protective effect of alpha-tocopheral on biochemical and histological alterations induced by cadmium in rat testes. Indian J. Physiol. Pharmacol. 2011, 55, 213–220. [Google Scholar] [PubMed]
- Mouro, V.G.S.; Siman, V.A.; da Silva, J.; Dias, F.C.R.; Damasceno, E.M.; Cupertino, M.d.C.; de Melo, F.C.S.A.; da Matta, S.L.P. Cadmium-induced testicular toxicity in mice: Subacute and subchronic route-dependent effects. Biol. Trace Elem. Res. 2020, 193, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Mouro, V.G.; Martins, A.L.; Silva, J.; Menezes, T.P.; Gomes, M.L.; Oliveira, J.A.; Melo, F.C.; Matta, S.L. Subacute testicular toxicity to cadmium exposure intraperitoneally and orally. Oxidative Med. Cell. Longev. 2019, 2019, 429635. [Google Scholar] [CrossRef] [Green Version]
- Momeni, H.; Eskandari, N. Curcumin protects the testis against cadmium-induced histopathological damages and oxidative stress in mice. Hum. Exp. Toxicol. 2020, 39, 653–661. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Cabaj, M.; Massanyi, P.; Martiniakova, M.; Omelka, R.; Krajcovicova, V.; Duranova, H. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. Sci. World J. 2014, 2014, 632581. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Li, X.; Ge, R.-S. Toxicological effects of cadmium on mammalian testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.H.; Zamiri, M.J.; Nazem, M.N.; Shirazi, M.R.J.; Akhlaghi, A.; Pirsaraei, Z.A. Detrimental effects of long-term exposure to heavy metals on histology, size and trace elements of testes and sperm parameters in Kermani Sheep. Ecotoxicol. Environ. Saf. 2021, 207, 111563. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.F.; Rattner, B.A. Ecotoxicology of Wild Mammals; Wiley: Chichester, UK, 2001. [Google Scholar]
- Zhou, B.; Gentry, A.; Xu, Q.; Young, J.L.; Yan, X.; Pagidas, K.; Yang, Y.; Watson, W.H.; Kong, M.; Cai, L. Effects of cadmium and high-fat diet on essential metal concentration in the mouse testis. Toxicol. Rep. 2021, 8, 718–723. [Google Scholar] [CrossRef] [PubMed]
- de Souza Predes, F.; Diamante, M.A.S.; Dolder, H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010, 91, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhu, Y.; Yang, Z.; Fu, S.; Zhang, W.; Liu, C. Protective effect of Polygonatum sibiricum against cadmium-induced testicular injury in mice through inhibiting oxidative stress and mitochondria-mediated apoptosis. J. Ethnopharmacol. 2020, 261, 113060. [Google Scholar] [CrossRef] [PubMed]
- Shojaeepour, S.; Dabiri, S.; Dabiri, B.; Imani, M.; Abadi, M.F.S.; Hashemi, F. Histopathological findings of testicular tissue following cadmium toxicity in rats. Iran. J. Pathol. 2021, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ma, Y.; Zhu, J.; Zou, H.; Liu, Z. Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility. Cells 2022, 11, 3601. [Google Scholar] [CrossRef]
- Gandhi, J.; Hernandez, R.J.; Chen, A.; Smith, N.L.; Sheynkin, Y.R.; Joshi, G.; Khan, S.A. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 2017, 25, 103–110. [Google Scholar] [CrossRef]
- Xuezhi, J.; Youxin, L.; Yilan, W. Studies of lead exposure on reproductive system: A review of work in China. Biomed. Environ. Sci. BES 1992, 5, 266–275. [Google Scholar]
- Vigeh, M.; Smith, D.R.; Hsu, P.-C. How does lead induce male infertility? Iran. J. Reprod. Med. 2011, 9, 1. [Google Scholar]
- Zhang, Z.; Yu, J.; Xie, J.; Liu, D.; Fan, Y.; Ma, H.; Wang, C.; Hong, Z. Improvement roles of zinc supplementation in low dose lead induced testicular damage and glycolytic inhibition in mice. Toxicology 2021, 462, 152933. [Google Scholar] [CrossRef]
- Elsheikh, N.A.H.; Omer, N.A.; Yi-Ru, W.; Mei-Qian, K.; Ilyas, A.; Abdurahim, Y.; Wang, G.L. Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 2020, 52, e13600. [Google Scholar] [CrossRef]
- Wahab, O.A.; Princely, A.C.; Oluwadamilare, A.A.; Oore-oluwapo, D.O.; Blessing, A.O.; Alfred, E.F. Clomiphene citrate ameliorated lead acetate-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2019, 23, 336. [Google Scholar] [PubMed]
- Kelainy, E.G.; Ibrahim Laila, I.M.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Pollut. Res. 2019, 26, 31675–31684. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological actions of androgens. Endocr. Rev. 1987, 8, 1–28. [Google Scholar] [CrossRef]
- Coffey, D. Androgen Action and the Sex Accessory Tissues. In The Physiology of Reproduction; Knobil, E., Neill, J.J., Ewing, L.L., Eds.; Raven Press: New York, NY, USA, 1988; pp. 1081–1118. [Google Scholar]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Padmanahban, V.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil. Steril. 2010, 93, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Y.; Feng, R.; Zheng, P.; Huang, H.; Zhou, S.; Ji, W.; Huang, F.; Liu, H.; Zhang, G. Cadmium induces testosterone synthesis disorder by testicular cell damage via TLR4/MAPK/NF-κB signaling pathway leading to reduced sexual behavior in piglets. Ecotoxicol. Environ. Saf. 2022, 233, 113345. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, B.; Young, J.L.; Wintergerst, K.; Cai, L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113373. [Google Scholar] [CrossRef]
- Wang, L.; Xun, P.; Zhao, Y.; Wang, X.; Qian, L.; Chen, F. Effects of lead exposure on sperm concentrations and testes weight in male rats: A meta-regression analysis. J. Toxicol. Environ. Health Part A 2008, 71, 454–463. [Google Scholar] [CrossRef]






| Cd (ug/g) | Pb (ug/g) | |||||
|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | |
| Plant A | 0.010 a | 0.014 a | 0.137 b | 0.714 a | 0.698 a | 2.283 b |
| Plant B | 0.008 a | 0.006 a | 0.089 b | 0.061 a | 0.083 a | 1.209 b |
| Plant C | 0.035 a | 0.007 b | 0.129 c | 0.235 a | 0.302 a | 2.091 b |
| Plant D | 0.010 a | 0.013 a | 0.110 b | 0.098 a | 0.116 a | 0.869 b |
| Plant E | 0.008 a | 0.010 a | 0.097 b | 0.074 a | 0.088 a | 0.938 b |
| Testes/Studies | Factor | Right Testes | Left Testes | Paired |
|---|---|---|---|---|
| Weight (g) | Site 1 | 94.7 ± 13.5 a | 92.3 ± 12.8 a | 187 ± 26.4 a |
| Site 2 | 93.9 ± 14.6 a | 90.9 ± 14.3 a | 185 ± 27.8 a | |
| Site 3 | 81.1 ± 12.5 b | 79.4 ± 11.2 b | 160.5 ± 22.5 b | |
| Length (mm) | Site 1 | 87.3 ± 12.4 c | 85.6 ± 11.4 c | |
| Site 2 | 88.2 ± 12.1 c | 84.8 ± 12.5 c | ||
| Site 3 | 74.8 ± 11.3 d | 74.2 ± 10.5 d | ||
| Width (mm) | Site 1 | 57.3 ± 11.8 e | 55.5 ± 14.3 e | |
| Site 2 | 55.4 ± 12.5 e | 56.6 ± 12.8 e | ||
| Site 3 | 45.4 ± 9.9 f | 44.3 ± 12.5 f | ||
| Volume (cm3) | Site 1 | 141.3 ± 14.3 g | 139.3 ± 13.9 g | |
| Site 2 | 140.7 ± 14.9 g | 141.0 ± 12.5 g | ||
| Site 3 | 127.2 ± 13.8 h | 125.4 ± 12.2 h |
| Groups | Number of Sections | Score | |||||
|---|---|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | 0 | ||
| Site 1 | 30 | (80%) 25 | (36%) 11 | (50%) 15 | (20%) 6 | (27%) 8 | (13%) 4 |
| Site 2 | 30 | (77%) 23 | (34%) 10 | (53%) 16 | (26%) 8 | (24%) 7 | (13%) 4 |
| Site 3 | 30 | (40%) 12 | (16%) 5 | (10%) 3 | (46%) 14 | (33%) 10 | (13%) 4 |
| Score | Description |
|---|---|
| 5 | Complete spermatogenesis with mature sperm cells |
| 4 | Some sperm cells, with a disorganized epithelium |
| 3 | Presence of few sperm (<5 to 10) |
| 2 | Absence of sperm cells, presence of spermatids |
| 1 | Absence of sperm cells, presence of a few spermatids |
| 0 | Absence of sperm cells or spermatids, presence of spermatocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Ennab, W.; Wei, Q.; Wang, C.; Quddus, A.; Mustafa, S.; Hadi, T.; Mao, D.; Shi, F. Impact of Cadmium and Lead Exposure on Camel Testicular Function: Environmental Contamination and Reproductive Health. Animals 2023, 13, 2302. https://doi.org/10.3390/ani13142302
Ullah S, Ennab W, Wei Q, Wang C, Quddus A, Mustafa S, Hadi T, Mao D, Shi F. Impact of Cadmium and Lead Exposure on Camel Testicular Function: Environmental Contamination and Reproductive Health. Animals. 2023; 13(14):2302. https://doi.org/10.3390/ani13142302
Chicago/Turabian StyleUllah, Saif, Wael Ennab, Quanwei Wei, Changfa Wang, Abdul Quddus, Sheeraz Mustafa, Tavakolikazerooni Hadi, Dagan Mao, and Fangxiong Shi. 2023. "Impact of Cadmium and Lead Exposure on Camel Testicular Function: Environmental Contamination and Reproductive Health" Animals 13, no. 14: 2302. https://doi.org/10.3390/ani13142302