Udder Ultrasonography of Dairy Cows: Investigating the Relationship between Echotexture, Blood Flow, Somatic Cell Count and Milk Yield during Dry Period and Lactation
Abstract
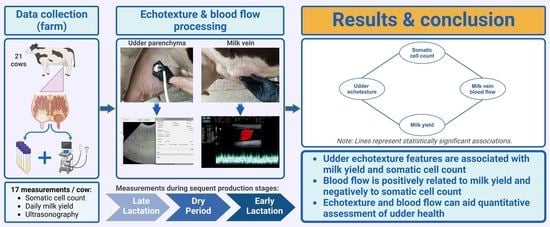
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design and Clinical Examination
2.3. Ultrasonography of the Milk Vein (B-Mode and Spectral Doppler)
2.4. Ultrasonography and Echotexture Analysis of the Mammary Parenchyma
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Blood Flow Volume, Daily Milk Yield and Somatic Cell Count
3.3. Udder Echotexture and Daily Milk Yield
3.4. Udder Echotexture and Somatic Cell Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Blood flow volume, daily milk yield and somatic cell count
- 2.
- Udder echotexture and daily milk yield
- 3.
- Udder echotexture and somatic cell score
References
- Caruolo, E.V.; Mochrie, R.D. Ultrasonograms of Lactating Mammary Glands. J. Dairy Sci. 1967, 50, 225–230. [Google Scholar] [CrossRef]
- Cartee, R.E.; Ibrahim, A.K.; McLeary, D. B-Mode Ultrasonography of the Bovine Udder and Teat. J. Am. Vet. Med. Assoc. 1986, 188, 1284–1287. [Google Scholar] [PubMed]
- Flöck, M.; Winter, P. Diagnostic Ultrasonography in Cattle with Diseases of the Mammary Gland. Vet. J. 2006, 171, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Floek, M.; Hofmann-Parisot, M. Ultrasonography of the Bovine Udder and Teat. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Gundling, N.; Drummer, C.; Lüpke, M.; Hoedemaker, M. Quantitative Measurement of Udder Oedema in Dairy Cows Using Ultrasound to Monitor the Effectiveness of Diuretic Treatment with Furosemide. Schweiz. Arch. Tierheilkd. 2021, 164, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Esselburn, K.M.; Hill, T.M.; Bateman, H.G.; Fluharty, F.L.; Moeller, S.J.; O’Diam, K.M.; Daniels, K.M. Examination of Weekly Mammary Parenchymal Area by Ultrasound, Mammary Mass, and Composition in Holstein Heifers Reared on 1 of 3 Diets from Birth to 2 Months of Age. J. Dairy Sci. 2015, 98, 5280–5293. [Google Scholar] [CrossRef]
- Albino, R.L.; Guimarães, S.E.F.; Daniels, K.M.; Fontes, M.M.S.; Machado, A.F.; dos Santos, G.B.; Marcondes, M.I. Technical Note: Mammary Gland Ultrasonography to Evaluate Mammary Parenchymal Composition in Prepubertal Heifers. J. Dairy Sci. 2017, 100, 1588–1591. [Google Scholar] [CrossRef]
- Franz, S.; Hofmann-Parisot, M.M.; Baumgartner, W. Evaluation of Three-Dimensional Ultrasonography of the Bovine Mammary Gland. Am. J. Vet. Res. 2004, 65, 1159–1163. [Google Scholar] [CrossRef]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture Analysis of Medical Images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef]
- Santos, V.; Simplício, K.; Sanchez, D.; Coutinho, L.; Teixeira, P.; Barros, F.; Almeida, V.; Rodrigues, L.; Bartlewski, P.; Oliveira, M.; et al. B-Mode and Doppler Sonography of the Mammary Glands in Dairy Goats for Mastitis Diagnosis. Reprod. Domest. Anim. 2015, 50, 251–255. [Google Scholar] [CrossRef]
- Schwarz, T.; Scheeres, N.; Małopolska, M.M.; Murawski, M.; Agustin, T.D.; Ahmadi, B.; Strzałkowska, N.; Rajtar, P.; Micek, P.; Bartlewski, P.M. Associations between Mammary Gland Echotexture and Milk Composition in Cows. Animals 2020, 10, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ahmad, M.J.; An, Z.; Niu, K.; Wang, W.; Nie, P.; Gao, S.; Yang, L. Relationship Between Somatic Cell Counts and Mammary Gland Parenchyma Ultrasonography in Buffaloes. Front. Vet. Sci. 2022, 9, 842105. [Google Scholar] [CrossRef] [PubMed]
- Ntemka, A.; Tsakmakidis, I.; Boscos, C.; Theodoridis, A.; Kiossis, E. The Role of Ewes’ Udder Health on Echotexture and Blood Flow Changes during the Dry and Lactation Periods. Animals 2022, 12, 2230. [Google Scholar] [CrossRef]
- Galloway, M.M. Texture Analysis Using Gray Level Run Lengths. Comput. Graph. Image Process. 1975, 4, 172–179. [Google Scholar] [CrossRef]
- Mailloux, G.E.; Bertrand, M.; Stampfler, R.; Ethier, S. Texture Analysis of Ultrasound B-Mode Images by Segmentation. Ultrason. Imaging 1984, 6, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Aschkenasy, S.V.; Muntwyler, J.; Van Der Loo, B.; Oechslin, E.; Jenni, R. Texture Analysis in Digitally-Acquired Echocardiographic Images: The Effect of JPEG Compression and Video Storage. Ultrasound Med. Biol. 2005, 31, 361–366. [Google Scholar] [CrossRef]
- Themistokleous, K.S.; Sakellariou, N.; Kiossis, E. A Deep Learning Algorithm Predicts Milk Yield and Production Stage of Dairy Cows Utilizing Ultrasound Echotexture Analysis of the Mammary Gland. Comput. Electron. Agric. 2022, 198, 106992. [Google Scholar] [CrossRef]
- Kronfeld, S.D.; Raggi, F.; Ramberg, C.F.J. Mammary Metabolism Blood Flow and Ketone Body in Normal, Fasted, and Ketotic Cows. Am. J. Physiol. 1968, 215, 218–227. [Google Scholar] [CrossRef]
- Gorewit, R.C.; Aromando, M.C.; Bristol, D.G. Measuring Bovine Mammary Gland Blood Flow Using a Transit Time Ultrasonic Flow Probe. J. Dairy Sci. 1989, 72, 1918–1928. [Google Scholar] [CrossRef]
- Delamaire, E.; Guinard-Flament, J. Increasing Milking Intervals Decreases the Mammary Blood Flow and Mammary Uptake of Nutrients in Dairy Cows. J. Dairy Sci. 2006, 89, 3439–3446. [Google Scholar] [CrossRef]
- Piccione, G.; Arcigli, A.; Fazio, F.; Giudice, E.; Caola, G. Pulsed Wave–Doppler Ultrasonographic Evaluation of Mammary Blood Flows Speed in Cows during Different Productive Periods. Acta Sci. Vet. 2004, 32, 171–175. [Google Scholar] [CrossRef]
- Götze, A.; Honnens, A.; Flachowsky, G.; Bollwein, H. Variability of Mammary Blood Flow in Lactating Holstein-Friesian Cows during the First Twelve Weeks of Lactation. J. Dairy Sci. 2010, 93, 38–44. [Google Scholar] [CrossRef]
- Potapow, A.; Sauter-Louis, C.; Schmauder, S.; Friker, J.; Nautrup, C.P.; Mehne, D.; Petzl, W.; Zerbe, H. Investigation of Mammary Blood Flow Changes by Transrectal Colour Doppler Sonography in an Escherichia coli Mastitis Model. J. Dairy Res. 2010, 77, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kjœrsgaard, P. Mammary Blood Flow and Venous Drainage in Cows. Acta Vet. Scand. 1974, 15, 179–187. [Google Scholar] [CrossRef]
- Braun, U.; Hoegger, R. B-Mode and Colour Doppler Ultrasonography of the Milk Vein in 29 Healthy Swiss Braunvieh Cows. Vet. Rec. 2008, 163, 47–49. [Google Scholar] [CrossRef]
- Braun, U.; Forster, E.; Bleul, U.; Hässig, M.; Schwarzwald, C. B-Mode and Colour Doppler Ultrasonography of the Milk Vein and Musculophrenic Vein in Eight Cows during Lactation. Res. Vet. Sci. 2013, 94, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Forster, E. B-Mode and Colour Doppler Sonographic Examination of the Milk Vein and Musculophrenic Vein in Dry Cows and Cows with a Milk Yield of 10 and 20 Kg. Acta Vet. Scand. 2012, 54, 15. [Google Scholar] [CrossRef]
- Rizzo, A.; Mutinati, M.; Minoia, G.; Spedicato, M.; Pantaleo, M.; Sciorsci, R.L. The Impact of Oxytocin on the Hemodynamic Features of the Milk Vein in Dairy Cows: A Color Doppler Investigation. Res. Vet. Sci. 2012, 93, 983–988. [Google Scholar] [CrossRef]
- Jackson, P.G.G.; Cockcroft, P.D. Clinical Examination of Farm Animals, 1st ed.; Blackwell Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Rossi, R.S.; Amarante, A.F.; Correia, L.B.N.; Guerra, S.T.; Nobrega, D.B.; Latosinski, G.S.; Rossi, B.F.; Rall, V.L.M.; Pantoja, J.C.F. Diagnostic Accuracy of Somaticell, California Mastitis Test, and Microbiological Examination of Composite Milk to Detect Streptococcus Agalactiae Intramammary Infections. J. Dairy Sci. 2018, 101, 10220–10229. [Google Scholar] [CrossRef] [PubMed]
- Barnouin, J.; Chassagne, M.; Bazin, S.; Boichard, D. Management Practices from Questionnaire Surveys in Herds with Very Low Somatic Cell Score Through a National Mastitis Program in France. J. Dairy Sci. 2004, 87, 3989–3999. [Google Scholar] [CrossRef]
- Kline, D.D.; Hasser, E.M.; Heesch, C.M. Regulation of the Heart. In Dukes’ Physiology of Domestic Animals, 13th ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2015. [Google Scholar]
- Schmauder, S.; Weber, F.; Kiossis, E.; Bollwein, H. Cyclic Changes in Endometrial Echotexture of Cows Using a Computer-Assisted Program for the Analysis of First- and Second-Order Grey Level Statistics of B-Mode Ultrasound Images. Anim. Reprod. Sci. 2008, 106, 153–161. [Google Scholar] [CrossRef]
- Molengerghs, G.; Verbeke, G. Models for Discrete Longitudinal Data; Springer Series in Statistics; Springer: New York, NY, USA, 2005; ISBN 0-387-25144-8. [Google Scholar]
- Harrell, F.E. Regression Modeling Strategies; Springer Series in Statistics; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-19424-0. [Google Scholar]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Oliver, S.P.; Sordillo, L.M. Udder Health in the Periparturient Period. J. Dairy Sci. 1988, 71, 2584–2606. [Google Scholar] [CrossRef]
- Dufour, S.; Dohoo, I.R. Monitoring Dry Period Intramammary Infection Incidence and Elimination Rates Using Somatic Cell Count Measurements. J. Dairy Sci. 2012, 95, 7173–7185. [Google Scholar] [CrossRef]
- Penry, J.F.; Crump, P.M.; Hernandez, L.L.; Reinemann, D.J. Association of Quarter Milking Measurements and Cow-Level Factors in an Automatic Milking System. J. Dairy Sci. 2018, 101, 7551–7562. [Google Scholar] [CrossRef]
- Tyler, J.W.; Thurmond, M.C.; Lasslo, L. Relationship between Test-Day Measures of Somatic Cell Count and Milk Production in California Dairy Cows. Can. J. Vet. Res. 1989, 53, 182–187. [Google Scholar]
- Koldeweij, E.; Emanuelson, U.; Janson, L. Relation of Milk Production Loss to Milk Somatic Cell Count. Acta Vet. Scand. 1999, 40, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Green, L.E.; Schukken, Y.H.; Green, M.J. On Distinguishing Cause and Consequence: Do High Somatic Cell Counts Lead to Lower Milk Yield or Does High Milk Yield Lead to Lower Somatic Cell Count? Prev. Vet. Med. 2006, 76, 74–89. [Google Scholar] [CrossRef]
- Hagnestam-Nielsen, C.; Emanuelson, U.; Berglund, B.; Strandberg, E. Relationship between Somatic Cell Count and Milk Yield in Different Stages of Lactation. J. Dairy Sci. 2009, 92, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ponchon, B.; Lanctôt, S.; Lacasse, P. Invited Review: Accelerating Mammary Gland Involution after Drying-off in Dairy Cattle. J. Dairy Sci. 2019, 102, 6701–6717. [Google Scholar] [CrossRef] [PubMed]
- Murawski, M.; Schwarz, T.; Jamieson, M.; Ahmad, B.; Bartlewski, P.M. Echotextural Characteristics of the Mammary Gland during Early Lactation in Two Breeds of Sheep Varying in Milk Yields. Anim. Reprod. 2019, 16, 853–858. [Google Scholar] [CrossRef] [PubMed]



| Late Lactation (N = 84) | Dry Period (N = 230) | Early Lactation (N = 376) | p-Value | ||
|---|---|---|---|---|---|
| D1 (cm) | Mean (SD) | 0.53 (0.14) | 0.55 (0.14) | 0.56 (0.18) | 0.287 |
| Median (IQR) | 0.52 (0.23) | 0.53 (0.19) | 0.52 (0.16) | ||
| D2 (cm) | Mean (SD) | 2.36 (0.39) | 2.31 (0.42) | 2.39 (0.42) | 0.591 |
| Median (IQR) | 2.36 (0.52) | 2.25 (0.58) | 2.37 (0.48) | ||
| A (cm2) | Mean (SD) | 5.85 (1.53) | 5.61 (1.60) | 5.90 (1.73) | 0.701 |
| Median (IQR) | 5.87 (2.27) | 5.44 (2.19) | 5.81 (2.00) | ||
| BFVol (L/min) | Mean (SD) | 5.26 (2.24) | 2.70 (1.30) | 7.19 (2.44) | <0.01 |
| Median (IQR) | 4.50 (3.32) | 2.40 (1.77) | 6.95 (3.42) | ||
| TAMV (cm/s) | Mean (SD) | 15.2 (5.60) | 8.15 (3.78) | 20.6 (5.88) | <0.01 |
| Median (IQR) | 15.0 (10.3) | 7.00 (5.00) | 20.0 (6.00) | ||
| Vmax (cm/s) | Mean (SD) | 47.8 (18.9) | 27.10 (11.9) | 63.9 (17.6) | <0.01 |
| Median (IQR) | 45.0 (32.2) | 24.0 (16) | 61.0 (20.2) |
| Outcome: BFVol (L/min) | Univariable Model’s Estimates (95% C.I.) 1 | p-Value | Multivariable Model’s Estimates (95% C.I.) 2 | p-Value |
|---|---|---|---|---|
| DMY (kg) | 0.06 * | <0.01 | 0.25 * | <0.01 |
| (0.03, 0.08) | (0.13, 0.39) | |||
| iSCC (cells/mL) | −0.49 * | 0.04 | −0.42 | 0.07 |
| (for 1,000,000-unit increase) | (−0.97, −0.01) | (−0.91, 0.05) | ||
| Lactation Period (Ref: 1st) | ||||
| 2nd LP | −0.03 | 0.948 | −0.07 | 0.913 |
| (−0.95, 0.90) | (−1.41, 1.25) | |||
| 3rd LP | 0.41 | 0.586 | 0.36 | 0.725 |
| (−1.07, 1.87) | (−1.70, 2.41) | |||
| 4th LP | −0.75 | 0.448 | −0.41 | 0.765 |
| (−2.95, 1.49) | (−4.41, 3.33) |
| Outcome: Daily Milk Yield (kg) | Univariable Model’s Estimates (95% C.I.) 1 | p-Value | Multivariable Model’s Estimates (95% C.I.) 1 | p-Value |
|---|---|---|---|---|
| Mean Numerical Pixel Value | 0.11 (0.05, 0.16) * | <0.01 | 0.09 (0.03, 0.15) * | 0.003 |
| Mean Pixel Standard Deviation | 0.72 (0.28, 1.18) * | <0.01 | 0.57 (0.11, 1,03) * | 0.01 |
| Gradient Mean Value | 0.30 (0.15, 0.47) * | <0.01 | ||
| Contrast | 0.007 (−0.01, 0.03) | 0.554 | ||
| Correlation 2 | −3.73 (−6.14, −1.48) * | <0.01 | ||
| Homogeneity 3 | −0.86 (−3.13, 1.40) | 0.454 | ||
| Run Percentage | 1.22 (−1.60, 4.04) | 0.397 | ||
| Gradient Variance 4 | 1.96 (−0.06, 3.98) | 0.057 | ||
| Grey Value Distribution 5 | −1.28 (−8.75, 6.18) | 0.736 | ||
| Runlength Distribution 5 | −1.16 (−8.72, 6.39) | 0.763 | ||
| Entropy | 0.59 (−4.98, 6.17) | 0.835 | ||
| Excess | −5.86 (−8.68, −3.05) * | <0.01 | ||
| Percentage Non-zero Gradients | −0.46 (−2.29, 1.36) | 0.619 | ||
| Long-Run Emphasis 3 | −0.15 (−0.33, 0.02) | 0.087 | −0.23 (−0.46, −0.002) * | 0.048 |
| Skewness | −4.25 (−6.27, −2.23) * | <0.01 |
| Outcome: Quarter Somatic Cell Score | Univariable Model’s Estimates (95% C.I.) 1 | p-Value | Multivariable Model’s Estimates (95% C.I.) 1 | p-Value |
|---|---|---|---|---|
| Mean Numerical Pixel Value | 0.005 (−0.01, 0.02) | 0.623 | −0.07 (−0.12, −0.02) * | <0.01 |
| Mean Pixel Standard Deviation | 0.27 (0.10, 0.44) * | <0.01 | 0.72 (0.43, 1.00) * | <0.01 |
| Gradient Mean Value | 0.04 (−0.02, 0.10) | 0.196 | 0.17 (0.05, 0.30) * | <0.01 |
| Contrast | 0.001 (−0.007, 0.010) | 0.725 | ||
| Correlation 2 | −1.18 (−2.02, −0.33) * | <0.01 | ||
| Homogeneity 3 | −0.13 (−0.97, 0.71) | 0.761 | −1.23 (−2.19, −0.28) * | 0.01 |
| Run Percentage | 0.27 (−0.79, 1.34) | 0.615 | ||
| Gradient Variance 4 | 0.05 (−0.71, 0.82) | 0.890 | −2.50 (−3.75, −1.26) * | <0.01 |
| Grey Value Distribution 5 | 0.36 (−2.42, 3.15) | 0.798 | ||
| Runlength Distribution 5 | 0.49 (−2.32, 3.31) | 0.731 | ||
| Entropy | −0.72 (−2.82, 1.36) | 0.493 | −4.36 (−6.95, −1.79) * | <0.01 |
| Excess | −0.52 (−1.57, 0.51) | 0.323 | ||
| Percentage Non-zero Gradients | 0.0005 (−0.68, 0.68) | 0.998 | ||
| Long-Run Emphasis 3 | −0.014 (−0.082, 0.053) | 0.677 | ||
| Skewness | −0.27 (−0.10, 0.46) | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Themistokleous, K.S.; Papadopoulos, I.; Panousis, N.; Zdragas, A.; Arsenos, G.; Kiossis, E. Udder Ultrasonography of Dairy Cows: Investigating the Relationship between Echotexture, Blood Flow, Somatic Cell Count and Milk Yield during Dry Period and Lactation. Animals 2023, 13, 1779. https://doi.org/10.3390/ani13111779
Themistokleous KS, Papadopoulos I, Panousis N, Zdragas A, Arsenos G, Kiossis E. Udder Ultrasonography of Dairy Cows: Investigating the Relationship between Echotexture, Blood Flow, Somatic Cell Count and Milk Yield during Dry Period and Lactation. Animals. 2023; 13(11):1779. https://doi.org/10.3390/ani13111779
Chicago/Turabian StyleThemistokleous, Konstantinos S., Iraklis Papadopoulos, Nikolaos Panousis, Antonios Zdragas, Georgios Arsenos, and Evangelos Kiossis. 2023. "Udder Ultrasonography of Dairy Cows: Investigating the Relationship between Echotexture, Blood Flow, Somatic Cell Count and Milk Yield during Dry Period and Lactation" Animals 13, no. 11: 1779. https://doi.org/10.3390/ani13111779