Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products
Abstract
:Simple Summary
Abstract
1. Introduction
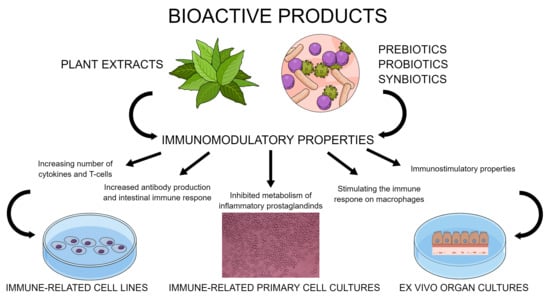
2. Immunomodulatory Properties of Bioactive Products
2.1. Plant Extracts
2.2. Prebiotics, Probiotics, and Synbiotics
2.3. Immunomodulatory Effects of Bioactive Compounds
3. Avian Cell Culture Models for Testing Immunomodulatory Effects of Bioactive Products
3.1. Immune-Related Primary Cells Cultures
3.2. Immune-Related Cell Lines in Chicken
3.3. Ex Vivo Organ Cultures
3.4. In Ovo Injections
3.5. In Vivo Models vs. In Vitro Models
3.6. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A Natural Feed Additive for Poultry Health and Production—A Review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Food Sci. Nutr. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Merriam-Webster Medical Dictionary. Available online: https://www.merriam-webster.com/medical/immunomodulation (accessed on 13 February 2020).
- Gea-Banacloche, J.C. Immunomodulation. In Principles of Molecular Medicine; Humana Press: Totowa, NJ, USA, 2006; pp. 893–904. ISBN 9781588292025. [Google Scholar]
- Haruna, A.; Yahaya, S.M. Recent Advances in the Chemistry of Bioactive Compounds from Plants and Soil Microbes: A Review. Chem. Afr. 2021, 4, 231–248. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, W. The application of probiotics in hatchery. Int. Hatch. Pract. 2017, 29, 11. [Google Scholar]
- Thaxton, J.P.; Parkhurst, C.R. Growth, Efficiency, and Livability of Newly Hatched Broilers as Influenced by Hydration and Intake of Sucrose. Poult. Sci. 1976, 55, 2275–2279. [Google Scholar] [CrossRef]
- Babot, J.D.; Argañaraz-Martínez, E.; Saavedra, L.; Apella, M.C.; Chaia, A.P. Selection of indigenous lactic acid bacteria to reinforce the intestinal microbiota of newly hatched chicken—Relevance of in vitro and ex vivo methods for strains characterization. Res. Vet. Sci. 2014, 97, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bierer, B.W.; Eleazer, T.H.; Barnett, B.D. The Effect of Feed and Water Deprivation on Water and Feed Consumption, Body Weight and Mortality in Broiler Chickens of Various Ages. Poult. Sci. 1966, 45, 1045–1051. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Glick, B.; Chang, T.S.; Jaap, R.G. The Bursa of Fabricius and Antibody Production. Poult. Sci. 1956, 35, 224–225. [Google Scholar] [CrossRef]
- Cooper, M.D.; Peterson, R.D.A.; Good, R.A. Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken. Nature 1965, 205, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Jansen, C.A.; Toyomizu, M.; van Eden, W. The Well-Developed Mucosal Immune Systems of Birds and Mammals Allow for Similar Approaches of Mucosal Vaccination in Both Types of Animals. Front. Nutr. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Korošec, T.F.; Voljč, M.; Salobir, J.; Rezar, V.; Frankič, T. Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 2009, 94, 95–102. [Google Scholar]
- Teodoro, A.J. Bioactive compounds of food: Their role in the prevention and treatment of diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A. Alternatives to Antibiotic Growth Promoters in Poultry and Pigs|The Poultry Site. Available online: https://www.thepoultrysite.com/articles/alternatives-to-antibiotic-growth-promoters-in-poultry-and-pigs (accessed on 10 February 2022).
- Hashemi, S.; Davoodi, H. Herbal plants an new immono-stimulator in poultry industry: A review. Asian J. Anim. Vet. Adv. 2012, 7, 105–116. [Google Scholar] [CrossRef]
- Sethiya, N.K. Review on Natural Growth Promoters Available for Improving Gut Health of Poultry: An Alternative to Antibiotic Growth Promoters. Asian J. Poult. Sci. 2016, 10, 1–29. [Google Scholar] [CrossRef]
- Huang, C.M.; Lee, T.T. Immunomodulatory effects of phytogenics in chickens and pigs—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauze, M. Phytobiotics, a Natural Growth Promoter for Poultry. In Advanced Studies in the 21st Century Animal Nutrition; IntechOpen: London, UK, 2021; pp. 1–21. ISBN 978-1-83969-404-2. [Google Scholar]
- Çabuk, M.; Bozkurt, M.; Alçiçek, A.; Akbaþ, Y.; Küçükyýlmaz, K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. S. Afr. J. Anim. Sci. 2006, 36, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lillehoj, H.S.; Hong, Y.H.; Jang, S.I.; Lillehoj, E.P.; Ionescu, C.; Mazuranok, L.; Bravo, D. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br. Poult. Sci. 2010, 51, 213–221. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lee, K.W.; Park, M.S.; Bravo, D.; Lillehoj, E.P. Cinnamaldehyde enhances in vitro parameters of immunity and reduces in vivo infection against avian coccidiosis. Br. J. Nutr. 2011, 106, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Lahlou, R.A.; Bounechada, M.; Mohammedi, A.; Silva, L.R.; Alves, G. Dietary use of Rosmarinus officinalis and Thymus vulgaris as anticoccidial alternatives in poultry. Anim. Feed Sci. Technol. 2021, 273, 114826. [Google Scholar] [CrossRef]
- Yang, W.C.; Yang, C.Y.; Liang, Y.C.; Yang, C.W.; Li, W.Q.; Chung, C.Y.; Yang, M.T.; Kuo, T.F.; Lin, C.F.; Liang, C.L.; et al. Anti-coccidial properties and mechanisms of an edible herb, Bidens pilosa, and its active compounds for coccidiosis. Sci. Rep. 2019, 9, 2896. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Kim, D.K.; Bravo, D.M.; Lee, S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 2011, 5, S34. [Google Scholar] [CrossRef] [Green Version]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical control of poultry coccidiosis: A review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef]
- Dankowiakowska, A.; Kozłowska, I.; Bednarczyk, M. Probiotics, prebiotics and snybiotics in Poultry–mode of action, limitation, and achievements. J. Cent. Eur. Agric. 2013, 14, 467–478. [Google Scholar] [CrossRef]
- Olędzki, R.; Hristova, A. Bioactive Components in Functional Products and Their Role in Human Nutrition. Eng. Sci. Technol. 2017, 1, 40–61. [Google Scholar] [CrossRef]
- Alloui, M.N.; Szczurek, W.; Światkiewicz, S. The usefulness of prebiotics and probiotics in modern poultry nutrition: A review. Ann. Anim. Sci. 2013, 13, 17–32. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO; WHO. Food and Agriculture Organization of the United Nations World Health Organization. In Guidelines for the Evaluation of Probiotics in Food, Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: London, UK; WHO: Ottawa, ON, Canada, 2002. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; Croom, W.J.; Edens, F.W.; McBride, B.W.; Qiu, R.; Chiang, C.C.; Daniel, L.R.; Havenstein, G.B.; Koci, M.D. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult. Sci. 2007, 86, 1121–1132. [Google Scholar] [CrossRef]
- Jung, B.G.; Ko, J.H.; Lee, B.J. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against salmonella Gallinarum in experimentally infected broiler chicks. J. Vet. Med. Sci. 2010, 72, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Sunu, P.; Sunarti, D.; Mahfudz, L.D.; Yunianto, V.D. Effect of synbiotic from Allium sativum and Lactobacillus acidophilus on hematological indices, antioxidative status and intestinal ecology of broiler chicken. J. Saudi Soc. Agric. Sci. 2021, 20, 103–110. [Google Scholar] [CrossRef]
- Al-Kassie, G.A.M. Influence of Two Plant Extracts Derived From Thyme and Cinnamon on. Nutrition 2009, 29, 169–173. [Google Scholar]
- Mumtaz, S.; Akhtar, M.; Awais, M.M.; Anwar, M.I. Evaluation of immunomodulatory, growth promoting and protective effects of ficus religiosa against coccidiosis in broilers. Pak. J. Agric. Sci. 2021, 58, 219–228. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Davidge, L.; Roshdieh, A.; Sharif, S. Characterization of the effects of three Lactobacillus species on the function of chicken macrophages. Res. Vet. Sci. 2015, 100, 39–44. [Google Scholar] [CrossRef]
- Djeraba, A.; Quere, P. In vivo macrophage activation in chickens with Acemannan, a complex carbohydrate extracted from Aloe vera. Int. J. Immunopharmacol. 2000, 22, 365–372. [Google Scholar] [CrossRef]
- Smith, J.M. A Review of Avian Probiotics. J. Avian Med. Surg. 2014, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Waihenya, R.K.; Mtambo, M.M.A.; Nkwengulila, G.; Minga, U.M. Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania. J. Ethnopharmacol. 2002, 79, 317–323. [Google Scholar] [CrossRef]
- Akhtar, M.; Tariq, A.F.; Awais, M.M.; Iqbal, Z.; Muhammad, F.; Shahid, M.; Hiszczynska-Sawicka, E. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohydr. Polym. 2012, 90, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J. Cell. Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Husáková, E.; Spišáková, V.; Herich, R.; Kolesárová, M.; Stašová, D.; Levkutová, M.; Levkut, M. Expression of cytokines in chicken peripheral mononuclear blood cells (PMBCS) exposed to probiotic strains and Salmonella enteritidis. Acta Vet. Brno 2015, 84, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Dalloul, R.A.; Lillehoj, H.S.; Shellem, T.A.; Doerr, J.A. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 2003, 82, 62–66. [Google Scholar] [CrossRef]
- Spivey, M.A.; Dunn-Horrocks, S.L.; Duong, T. Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult. Sci. 2014, 93, 2910–2919. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef] [Green Version]
- Slawinska, A.; Dunislawska, A.; Plowiec, A.; Gonçalves, J.; Siwek, M. TLR-Mediated Cytokine Gene Expression in Chicken Peripheral Blood Mononuclear Cells as a Measure to Characterize Immunobiotics. Genes 2021, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Cai, D.; He, T.; Deng, L.; Wu, L.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; et al. Isolation and Selection of Duck Primary Cells as Pathogenic and Innate Immunologic Cell Models for Duck Plague Virus. Front. Immunol. 2020, 10, 3131. [Google Scholar] [CrossRef]
- Yuan, C.; He, Q.; Li, J.M.; Azzam, M.M.; Lu, J.J.; Zou, X.T. Evaluation of embryonic age and the effects of different proteases on the isolation and primary culture of chicken intestinal epithelial cells in vitro. Anim. Sci. J. 2015, 86, 588–594. [Google Scholar] [CrossRef]
- Li, G.H.; Hong, Z.M.; Jia, Y.J.; You, J.M.; Zhang, J.H.; Liu, B.S. Probiotic Lactobacilli stimulate avian beta-defensin 9 expression in cultured chicken small intestinal epithelial cells. Proc. Nutr. Soc. 2012, 71, E239. [Google Scholar] [CrossRef] [Green Version]
- Ghiselli, F.; Rossi, B.; Felici, M.; Parigi, M.; Tosi, G.; Fiorentini, L.; Massi, P.; Piva, A.; Grilli, E. Isolation, culture, and characterization of chicken intestinal epithelial cells. BMC Mol. Cell Biol. 2021, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rothwell, L.; Young, J.R.; Kaufman, J.; Butter, C.; Kaiser, P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 2010, 129, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Van den Biggelaar, R.H.G.A.; van der Maas, L.; Meiring, H.D.; Pennings, J.L.A.; van Eden, W.; Rutten, V.P.M.G.; Jansen, C.A. Proteomic analysis of chicken bone marrow-derived dendritic cells in response to an inactivated IBV + NDV poultry vaccine. Sci. Rep. 2021, 11, 12666. [Google Scholar] [CrossRef]
- Matulova, M.; Rajova, J.; Vlasatikova, L.; Volf, J.; Stepanova, H.; Havlickova, H.; Sisak, F.; Rychlik, I. Characterization of Chicken Spleen Transcriptome after Infection with Salmonella enterica Serovar Enteritidis. PLoS ONE 2012, 7, e48101. [Google Scholar] [CrossRef] [PubMed]
- Matulova, M.; Varmuzova, K.; Sisak, F.; Havlickova, H.; Babak, V.; Stejskal, K.; Zdrahal, Z.; Rychlik, I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet. Res. 2013, 44, 37. [Google Scholar] [CrossRef] [Green Version]
- Koenen, M.E.; Van Der Hulst, R.; Leering, M.; Jeurissen, S.H.M.; Boersma, W.J.A. Development and validation of a new in vitro assay for selection of probiotic bacteria that express immune-stimulating properties in chickens in vivo. FEMS Immunol. Med. Microbiol. 2004, 40, 119–127. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, T.W.; Giroir, B.P.; Humphries, E.H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology 1985, 144, 139–151. [Google Scholar] [CrossRef]
- Sławińska, A.; Siwek, M.; Bednarczyk, M. In vitro screening of immunomodulatory properties of synbiotics in chicken DT40 cell line. Anim. Sci. Pap. Rep. 2016, 34, 81–94. [Google Scholar]
- Molnár, J.; Póti, Á.; Pipek, O.; Krzystanek, M.; Kanu, N.; Swanton, C.; Tusnády, G.E.; Szallasi, Z.; Csabai, I.; Szüts, D. The genome of the chicken DT40 bursal lymphoma cell line. G3 Genes Genomes Genet. 2014, 4, 2231–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunislawska, A.; Slawinska, A.; Siwek, M. Expression of FOXJ1 and ITGB4 is Activated upon KLH and LTA Stimulation in the DT40 Cell Line. Folia Biol. 2017, 65, 9–18. [Google Scholar] [CrossRef]
- Beug, H.; von Kirchbach, A.; Döderlein, G.; Conscience, J.F.; Graf, T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979, 18, 375–390. [Google Scholar] [CrossRef]
- Babu, U.S.; Sommers, K.; Harrison, L.M.; Balan, K.V. Effects of fructooligosaccharide-inulin on Salmonella-killing and inflammatory gene expression in chicken macrophages. Vet. Immunol. Immunopathol. 2012, 149, 92–96. [Google Scholar] [CrossRef]
- Ibuki, M.; Kovacs-Nolan, J.; Fukui, K.; Kanatani, H.; Mine, Y. β 1-4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 2011, 139, 289–295. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nomura, K.; Hirayama, Y.; Kitagawa, T. Establishment and Characterization of a Chicken Hepatocellular Carcinoma Cell Line, LMH. Cancer Res. 1987, 47, 4460–4464. [Google Scholar]
- Kolenda, R.; Burdukiewicz, M.; Wimonc, M.; Aleksandrowicz, A.; Ali, A.; Szabo, I.; Tedin, K.; Scott, J.B.; Pickard, D.; Schierack, P.; et al. Identification of Natural Mutations Responsible for Altered Infection Phenotypes of Salmonella enterica Clinical Isolates by Using Cell Line Infection Screens. Appl. Environ. Microbiol. 2021, 87, e02177-20. [Google Scholar] [CrossRef]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000, 24, 325–342. [Google Scholar] [CrossRef]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk HHS Public Access. Cell 2017, 168, 1135–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, T.J.; Morris, K.M.; Mabbott, N.A.; Vervelde, L. Inside-out chicken enteroids with leukocyte component as a model to study host–pathogen interactions. Commun. Biol. 2021, 4, 377. [Google Scholar] [CrossRef]
- Pierzchalska, M.; Panek, M.; Czyrnek, M.; Grabacka, M. The three-dimensional culture of epithelial organoids derived from embryonic chicken intestine. Methods Mol. Biol. 2016, 1576, 135–144. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, S.Y.; Li, R.X.; Lin, X.; Mi, Y.L.; Zhang, C.Q. Culture and characterization of chicken small intestinal crypts. Poult. Sci. 2018, 97, 1536–1543. [Google Scholar] [CrossRef]
- Beaumont, M.; Blanc, F.; Cherbuy, C.; Egidy, G.; Giuffra, E.; Lacroix-Lamandé, S.; Wiedemann, A. Intestinal organoids in farm animals. Vet. Res. 2021, 52, 33. [Google Scholar] [CrossRef]
- Aldhous, M.C.; Shmakov, A.N.; Bode, J.; Ghosh, S. Characterization of conditions for the primary culture of human small intestinal epithelial cells. Clin. Exp. Immunol. 2001, 125, 32–40. [Google Scholar] [CrossRef]
- Udden, S.M.N.; Waliullah, S.; Harris, M.; Zaki, H. The ex vivo colon organ culture and its use in antimicrobial host defense studies. J. Vis. Exp. 2017, e55347. [Google Scholar] [CrossRef]
- Gratz, S.; Mykkänen, H.; El-Nezami, A.H. Aflatoxin B1 Binding by a Mixture of Lactobacillus and Propionibacterium: In vitro Versus Ex vivo. J. Food Prot. 2005, 68, 2470–2474. [Google Scholar] [CrossRef]
- Hamida, F.; Wiryawan, K.G.; Meryandini, A. Selection of lactic acid bacteria as probiotic candidate for chicken. Media Peternak. 2015, 38, 138–144. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Menten, J.F.M.; Silveira, H.; Melo, A.D.B.; Rostagno, M.H. Hops β-acids (Humulus lupulus) decrease intestinal gene expression of proinflammatory cytokines in an ex-vivo model DESCRIPTION OF PROBLEM. J. Appl. Poult. Res. 2016, 25, 191–196. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahi, A.; Mozhdeh, M.; Khabisi, M.; Seyed, N.; Mousavi, N. Optimum time of in ovo injection in eggs of young broiler breeder flock. In Proceedings of the 18th European Symposium on Poultry Nutrition, Poster Presentations, Cesme, Izmir, Turkey, 31 October–4 November 2011. [Google Scholar]
- Uni, Z.; Ferket, P.R.; Tako, E.; Kedar, O. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 2005, 84, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.A.; Alqhtani, A.H.; Elliott, K.E.C.; Bello, A.; Levy, A.W.; Peebles, E.D. Improvement in the performance and inflammatory reaction of Ross 708 broilers in response to the in ovo injection of 25-hydroxyvitamin D3. Poult. Sci. 2021, 100, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Choi, I.; Yun, C.H. Immunosecurity: Immunomodulants enhance immune responses in chickens. Anim. Biosci. 2021, 34, 321–337. [Google Scholar] [CrossRef]
- Zhai, W.; Neuman, S.; Latour, M.A.; Hester, P.Y. The Effect of In Ovo Injection of l-Carnitine on Hatchability of White Leghorns. Poult. Sci. 2008, 87, 569–572. [Google Scholar] [CrossRef]
- Zhai, W.; Rowe, D.E.; Peebles, E.D. Effects of commercial in ovo injection of carbohydrates on broiler embryogenesis. Poult. Sci. 2011, 90, 1295–1301. [Google Scholar] [CrossRef]
- El-Kholy, K.H.; Sarhan, D.M.A.; El-Said, E.A. Effect of In-ovo Injection of Herbal Extracts on Post-hatch Performance, Immunological, and Physiological Responses of Broiler Chickens. J. Worlds Poult. Res. 2021, 11, 183–192. [Google Scholar] [CrossRef]
- Chen, C.; White, D.L.; Marshall, B.; Kim, W.K. Role of 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 in Chicken Embryo Osteogenesis, Adipogenesis, Myogenesis, and Vitamin D3 Metabolism. Front. Physiol. 2021, 12, 637629. [Google Scholar] [CrossRef]
- Williams, C. In ovo vaccination and chick quality. Int. Hatch. Pract. 2005, 19, 7–13. [Google Scholar]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics—In ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.A. Organ Culture of Lymphoid Cells. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1899–1902. [Google Scholar]
- Leon, O. Effective antibiotic-free broiler breeder production. Int. Hatch. Pract. 2019, 33, 15–16. [Google Scholar]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.B.; Santone, K.S.; Acosta, D. Evaluation of cytotoxicity in cultured cells by enzyme leakage. J. Tissue Cult. Methods 1980, 6, 113–116. [Google Scholar] [CrossRef]
- Ekwall, B. Screening of Toxic Compounds in Mammalian Cell Cultures. Ann. N. Y. Acad. Sci. 1983, 407, 64–77. [Google Scholar] [CrossRef]
- Mia, M.M.; Hasan, M.; Miah, M.M.; Hossain, M.A.S.; Islam, S.E.; Islam, S.; Shanta, R.N. Inhibitory Potentiality of Secondary Metabolites Extracted from Marine Fungus Target on Avian Influenza Virus-A Subtype H5N8 (Neuraminidase) and H5N1 (Nucleoprotein): A Rational Virtual Screening. Vet. Anim. Sci. 2022, 15, 100231. [Google Scholar] [CrossRef]
| Bioactive Compound | Amount | Bird Models | Results | Reference |
|---|---|---|---|---|
| Arabinoxylan wheat bran (AXs) | Group A: AXs 100 mg/kg body weight/day Group B: AXs 200 mg/kg body weight/day Group C: AXs 300 mg/kg body weight/day | Industrial broiler chicks (Hubbard) | The results indicated a higher amount of anti-SRBC IgM in chickens in the experimental group compared to the control group. In the case of the amount of anti-SRBC IgG, it was also significantly higher in the experimental group than in the control group. | Adapted from Akhtar et al., 2012 [50] |
| Acemannan (ACM 1), a complex carbohydrate extracted from Aloe vera | 500 μg ACM vaccinated intramuscularly (6 chickens) and seemingly vaccinated (6 chickens) 3 days and 9 days before experimental analysis | 2-month-old White Leghorn chickens homozygous for the main histocompatibility haplotype B13 | ACM 1 permanently and effectively increased the activation capacity of macrophages from the systemic immune compartment (especially from the blood and spleen after intramuscular injection) in chickens, especially for the production of NO. | Adapted from Djerba et al., 2000 [47] |
| Thyme oil extract | 100 and 200 ppm (parts per milion) in the diet | 1 day-old broiler chicks of mixed-sex Arbor-Acres | Thyme improved weight gain, feed intake, and feed conversion rate, improving the digestive system. Chickens fed thyme oil extract had lower cholesterol levels and higher red blood cells, packed cell volume, hemoglobin, and white blood cells. | Adapted from Al-Kassie. 2009 [44] |
| Ficus religiosa | I. Aqueous extract—100, 200, 300 mg/kg body weightII. Ethanol extract—100, 200, 300 mg/kg body weight | 1-day broiler chicks–Cobb | Both types of extracts affected the immune system by improving cellular immune performance. The researchers also noted growth-promoting effects. | Adapted from Mumtaz et al., 2021 [45] |
| Combination of herbs fermented with probiotics: Curcuma longa, Houttuynia cordata, Prunus mume, and Rubus coreanus | Chickens in the experimental groups received the same feed containing 1% or 2% of a combination of fermented probiotics | 20-day-old Ross broiler chicks from one healthy Salmonella-free parent herd | The combination of probiotic-fermented herbs increased immune activity in broiler chicks such as antibody production level in serum and increased survival against Salomenlla gallinarum in experimentally infected broiler chickens due to stimulation of a nonspecific immune response. | Adapted from Jung et al., 2010 [41] |
| Sacharomyces boulardii and Bacillus subtilis | 1 × 106 cfu/mL for 3-, 6- and 12-h S. boulardii, B. subtilis and coculture S. boulardii and B. subtilis, 1 μg/mL lipopolysaccharide, saline phosphate buffer added to the control group | Chinese crossed chickens—dendritic cells derived from chicken bone marrow | The treatment groups modulated the phenotype and biological functions of chi-BMDC. Upstream levels of MHC-II, CD40, CD80, and CD86 gene expression in the stimulated groups, toll-like receptors TLR1, TLR2, TLR4, and chicken-specific TLR15 expressions improved, and the accompanying factors myD88, TRAF6, TAB1, and NFk-B increased in all treatment groups compared to control. The NFk-B response was significantly higher in the treatment of LPS in all groups. In addition, IL-1β, IL-17, IL-4, TGF-β, and IL-10 contrast, the LPS groups showed a marked increase in IL-12, INF-γ, and IL-8 concentration levels compared to the control group. | Adapted from Rajput et al., 2014 [51] |
| Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus salivarius | 1 × 106 CFU thermally killed S. typhimurium, live L. acidophilus, live L. reuteri, and live L. salivarius | 22 commercial broiler chicks of mixed-sex at the age of 5 or 6 weeks. The mononuclear cells of the spleen and the tonsils of the spleen were isolated and cultured | The three lactobacilli induced a much higher expression of interleukin 1β in spleen cells than in cecal tonsil cells—more inflammatory response in the spleen than in the cecal tonsil cells. L. acidophilus was more effective in inducing T-helper-1 cytokines, while L. salivarius induced a more anti-inflammatory response. | Adapted from Brisbin et al., 2010 [42] |
| E. faecium AL41, E. faecium 31, L. fermentum AD1 and infected Salmonella enterica serovar Enteritidis (SE147) | 200 μg E. faecium AL41, E. faecium H31, L. fermentum AD1 and Salmonella enterica infected serovar Enteritidis (SE147) 1 × 109 cfu/ml | Healthy poultry reared under standard conditions—peripheral mononuclear blood cells (PMBC) | The results showed that E. faecium AL41 exhibited the highest immunostimulating effect on the expression of selected cytokines by PMBC from chickens after Salmonella infection. | Adapted from Husáková et al., 2015 [52] |
| Probiotic based on Lactobacillus | Probiotic added in the amount of 1 g/kg of feed | 100-day broiler chicks Ross 308 | The results indicate that probiotic bacteria influenced the local immune response characterized by altered subpopulations of gut intraepithelial lymphocytes and increased birds’ resistance to E. acervulina, reflecting reduced oocyst shedding. | Adapted from Dalloul et al., 2013 [53] |
| In Vitro Model | Cell Description | Tested Factor | Conditions of Maintaining and Stimulation | Results | References |
|---|---|---|---|---|---|
| Primary Cell Cultures | |||||
| Cecal tonsils mononuclear cells | Tissues (spleen and cecal tonsils) crushed on a 40-µm nylon cell strainer to obtain single-cell suspension and separated into mononuclear cells with Histopaque–1077 | Lactobacillus acidophilus, Lb. reuteri, Lb. salivarius | 41 °C and 5% moisturized CO2 incubator; stimulation for 3, 6, 12 and 18 h; PMI medium with 10% fetal bovine serum and 200 U/mL penicillin, 80 g/mL streptomycin, and 25 mg gentamicin | Probiotics such as live lactobacilli induced expression of IL-1β, IL-12p40, IFN-γ, IL-18, IL-10, and TGF-β. | Adapted from Brisbin et al., 2010 [42] |
| Mononuclear cells of the spleen | Unicellular suspensions were isolated from the spleen. Mononuclear cells were obtained using the Ficoll-Paque gradient | L. paracasei, L. reuteri, L. brevis, L. plantarum, L. paracasei, L. murinus-animalis, L. buchneri, L. paracasei | 41 °C and 5% CO2; RPMI 1640 medium containing 10% chicken serum, 1% non-etheric amino acids, 1% L-glutamine, 1% streptomycin | Bacteria strains which had positive influence on in vitro proliferation of mononuclear cells of the spleen also had positive influence on specific humoral immune responses. | Adapted from Koenen et al., 2004 [65] |
| chBMDC | Chicken bone marrow dendritic cells | Saccharomyces boulardii, Bacillus subtilis | Maintained after obtaining cell culture for 6 days at 41 °C and 5% CO2; stimulation for 3.6 × 12 h; RPMI 1640 medium with 10% poultry serum, 1% nonessential amino acids, 1% L-glutamine, 1% streptomycin | Saccharomyces boulardii and Bacillus subtilis had an influence on TLR-mediated signaling to induce immunity in chBMDCs. | Adapted from Rajput et al., 2014 [51] |
| IEC | Intestinal epithelial cells | Lactobacillus fermentum, Lb. rhamnosus, Lb. rhamnosus, Lb. plantarum, Lb. ramous | 37 °C, 5% CO2, and 95% humidity; DMEM supplemented with 5% fetal calf serum, 2 mmol/l L-glutamine, 20 ng/mL, mouse epidermal growth factor, 2 μg/mL insulin from the bovine pancreas, 100 U/mL penicillin-streptomycin | There were changes in AvBD9 expression between probiotic bacteria and various bacteria stimulation doses. The Lactobacillus ramous MLGA strain had the strongest potential to increase AvBD9 expression of all the lactobacillus strains studied. | Adatpted from Li et al., 2012 [59] |
| PBMC | Peripheral mononuclear blood cells. The culture came from blood taken from the wing vein of chicks | probiotic fermented combination of four herbs delivered against Salmonella | 41 °C and 5% CO2; RPMI-1640 medium with 2% antibiotic-antimycotic. | The probiotic fermented combination of four herbs enhanced the immune activity in broiler chicken and increased the survivability against Salmonella. | Adapted from Jung et al., 2010 [41] |
| Enterococcus faecium, E. faecium, Lactobacillus fermentum | 39.5 °C and 5% CO2; RPMI 1640 medium with 10 mM and 10% FBS | Compared to other tested probiotics, E. faecium AL41 showed the highest immunostimulatory effect on the level of relative expression of selected cytokines and chemokines after Salmonella infection. | Adapted from Husáková et al., 2015 [52] | ||
| LPS, CpG ODN (short, synthetic, single-stranded DNA molecules, containing unmethylated CpG motifs), Pam3CSK4 (bacterial lipoproteins), Zymosan (S. cerevisiae), GOS (galactooligosaccharides), L. lactis subsp. cremoris, S. cerevisiae | 41.5–42.5 °C and 5% CO2; RPMI 1640 medium; 10% FBS, 1% GlutaMAX, 1% antibiotic-antimycotic (next phases cell culture without this substance); stimulation for 3, 6, and 9 h | L. lactis subsp. cremoris had immunostimulatory properties in chicken PBMC and were expressed by increased mRNA abundance of IL-1β, IL-6, IL-8, and IL-12p40. | Adapted from Slawinska et al., 2021 [56] | ||
| In Vitro Model | Cell Description | Tested Factor | Conditions of Maintenance and Stimulation | Results | References |
|---|---|---|---|---|---|
| Cell Lines | |||||
| DT40 | Cell line B established from bursa lymphoma infected with avian retrovirus RAV-1 | prebiotics: RFO, inulin, Bi2tos; probiotics: Lactococcus lactis subsp. lactis, L. lactis subsp. cremoris | Incubator CO2 37 °C and 5%; stimulation for 9 h; 80% advanced RPMI 1640 medium, 20% fetal bovine serum with 1 mM sodium pyruvate, 2 mM L-glutamine, 4.5 g/L glucose, 100 U/mL penicillin, 100 μg/mL streptomycin and 50 μM mercaptoethanol | The combination of prebiotic inulin and probiotic Lactococcus lactis subsp. lactis SL2 provided the strongest regulation of genes associated with the immune system, which proves the immunostimulating potential of this synbiotic. | Adapted from Sławinska et al., 2016 [69] |
| LPS, LTA, KLH antigens | Advanced RPMI 1640 medium with 20% fetal bovine serum and addition of sodium pyruvate, L-glutamine, glucose, penicillin, streptomycin, mercaptoethanol; 37 °C and 5% CO2; stimulation was carried out for 3, 6, 9, and 24 h | At 24 h after stimulation, KLH and LTA antigens significantly increased mRNA expression of the FOXJ1 and ITGB4 genes. LPS was not a powerful stimulator of the genes of interest. | Adapted from Dunislawska et al., 2017 [70] | ||
| HD11 | macrophage-like chicken cell line | FOS-inulin | Cells grown overnight at 41 °C and 5% CO2; the cells starved in a serum-free medium for 2 h, stimulated for 5 h; RPMI 1640 medium with 8% thermally inactivated chicken serum and 4% thermally inactivated fetal bovine serum, antibiotics, glutamine, and nonessential amino acids | FOS-inulin has the ability to modulate the innate immune system, which shows increased Salmonella Enteritidis killing and decreased organ colonization by these bacteria. | Adapted from Babu et al., 2012 [72] |
| MQ-NCSU | macrophage-like cell line derived from spleen cells of Leghorn hens challenged with the strain of Marek’s disease virus | b 1-4 mannobiose (MNB) | 41 °C and 5% CO2; RPMI 1640 medium with 8% FBS, 10% poultry serum, 5% tryptose phosphate broth and 50 g/mL penicillin-streptomycin, 5 × 10−5 M 2-mercaptoethanol | MNB’s ability to up-regulate the expression of genes involved in host defense and stimulate the formation of reactive oxygen and nitrogen species suggests that it can increase macrophages’ Salmonella-killing activity and may operate as a potent immunomodulator. | Adapted from Ibuki et al., 2011 [73] |
| LMH | epithelial cell line derived from hepatocellular carcinoma | Rimfampicin-Resistant Lactobacillus cultures (strains of Lactobacillus crispatus, Lb. gallinarum, Bacillus subtilis) | 37 °C and 5% CO2; Waymouth’s MB 752/1 medium with 10% FBS | Variables other than adhesion, such as bile tolerance, have a role in lactobacilli persistence in the gastrointestinal tract of chicken. | Adapted from Spivey et al., 2014 [54] |
| CHIC-8E11 (MM-CHiC clone 8E11) | intestinal epithelial cells obtained from chicken | Salmonella enterica | 37 °C and 5% CO2; Modified Eagle Dulbecco Medium (DMEM)/Ham’s F-12 with L-glutamine, penicillin-streptomycin, and bovine serum | This research showed that most isolates have similar infection phenotypes, and that isolates with different infection phenotypes can be used to find new genes or gene variations that influence epithelial infection, such as novel components involved in Salmonella adherence and the invasion of epithelial cells. | Adapted from Kolenda et al., 2021 [75] |
| Tissue | Purpose | Tested Factor | Culture Dish, Medium and Duration | Results | References |
|---|---|---|---|---|---|
| Chicken duodenal loops | Study of the probiotic’s ability to bind AFB1 | Lactobacillus rhamnosus LC-705, Propionibacterium freudenreichii subsp. shermanii JS | Falcon tube PBS, 1 to 30 min | The findings of this study clearly revealed that the probiotic mixture can bind AFB1 in vitro, slow down AFB1 absorption in the chick duodenum, and lower AFB1 levels in duodenal tissue ex vivo. | Adapted from Gratz et al., 2005 [84] |
| Chicken ileum | Investigate the properties of adherent joints in the intestines | Six LAB isolates (E1223, E3, E4, E5, E7, and E8) derived from spontaneously fermented maize | Falcon tube, PBS, 30 min | Antibiotic-resistant LAB isolates E5, E7, and E8 were able to stick to chicken ileal cells in vitro. E8 isolate performed better than E5 and E7 isolates. The isolates E5, E7, and E8 were 99% identical to the Pediococcus pentosaceus ATCC 25,745 strain. | Adapted from Hamida et al., 2015 [85] |
| Chicken ileum | Evaluation of the effect of hops β and lipopolysaccharide on cytokine gene expression | β-acid hops, lipopolysaccharide | Falcon tube, DMEM, 30 min | The study demonstrated the anti-inflammatory action of hops β-acids and, as a result, the potential immunomodulatory activity exerted on host tissue, since they were able to reduce the expression of proinflammatory cytokines even when an inflammatory producing substance was present (i.e., LPS). | Adapted from Bortoluzzi et al., 2016 [86] |
| Chicken ileum | Isolate and characterize lactic acid bacteria from poultry | Lactic acid bacteria of poultry origin isolated from the intestines of chickens and broilers | Falcon tube, RPMI 1640 supplemented with 1% fetal bovine serum 1 h | Six poultry LAB strains were found to have suitable in vitro probiotic properties. | Adapted from Reuben et al., 2019 [87] |
| Chicken intestinal epithelial cells | Isolation and culture | No factor to be tested, attempt to obtain and maintain IEC | 24-well plates with Matrigel matrix, DMEM | The research proved that chicken intestinal epithelial cells can be isolated and maintained in culture, and that they can be used as an in vitro intestinal model for future research. | Adapted from Ghiselli et al., 2021 [60] |
| 3D chicken enteroids from intestinal embryonic villi and adult crypts | Developing a model for host-pathogen interactions study | No factor to be tested, attempt to culture avian enteroids with multiple villus-crypt structures | Matrigel, Advanced DMEM/F12 supplemented with 10 mM HEPES, 2 mM L-Glutamine, 50 U/mL Penicillin/Streptomycin and 2% B27 supplement | The authors developed a procedure for differentiating leukocyte-containing avian enteroids with an accessible epithelial layer. These enteroids mimic their in vivo counterparts’ 3D architecture, polarity, barrier function, and cellular composition, making them a good in vitro model of the post-hatch and mature chicken gut. | Adapted from Nash et al., 2021 [78] |
| Intestinal organoids | Establishment of 3D culture of intestinal organoids | No tested factor, protocol for 3D of epithelial organoids | Matrigel; DMEM/Ham’s F12, GlutaMAX, antibiotic-antimicotic solution, insuin-transferrin-selenium reagent, human recombinant R-spondin 1, human recombinant Noggin, AEGF, PGE2 | The method of intestinal organoid culture derived from intestinal tissue fragments extracted from 18- to 20-day-old chicken embryos and placed in Matrigel was effectively introduced by the authors. | Adapted from Pierzchalska et al., 2016 [79] |
| Establishment of isolation and culture method for chicken small intestinal organoids | Various concentrations of growth factors in order to obtain optimal conditions for culture | Matrigel 3-D culture system; BASIC MEDIUM: DMEM/F12 culture medium (containing 10 mM HEPES, 100 U/mL penicillin, 100 mg/mL streptomycin, 20 mg/mL nystatin, and 2 mM glutamax, pH 7.4) and 3 groups containing various additives to the medium | This study showed that a culture medium containing 50 ng/mL EGF, 100 ng/mL Noggin, and 500 ng/mL R-spondin 1 may effectively enhance the growth of chicken intestine organoids in vitro. | Adapted from Li et al., 2018 [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradowska, M.; Dunislawska, A.; Siwek, M.; Slawinska, A. Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products. Animals 2022, 12, 670. https://doi.org/10.3390/ani12050670
Paradowska M, Dunislawska A, Siwek M, Slawinska A. Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products. Animals. 2022; 12(5):670. https://doi.org/10.3390/ani12050670
Chicago/Turabian StyleParadowska, Michelle, Aleksandra Dunislawska, Maria Siwek, and Anna Slawinska. 2022. "Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products" Animals 12, no. 5: 670. https://doi.org/10.3390/ani12050670