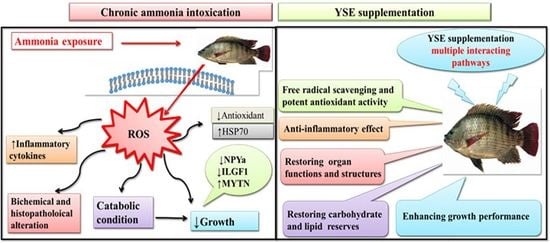
Exploring the Multimodal Role of Yucca schidigera Extract in Protection against Chronic Ammonia Exposure Targeting: Growth, Metabolic, Stress and Inflammatory Responses in Nile Tilapia (Oreochromis niloticus L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Fish, Diet and Experimental Design
2.3. Water Quality
2.4. Blood Sampling, Hematological and Serum Biochemical Analysis
2.5. Evaluation of Lipid Peroxidation and Antioxidant Enzymes
2.6. Histopathology Study
2.7. Total RNA Extraction, cDNA Synthesis and Real-Time Quantitative PCR Assay
2.8. Statistical Analyses of Data
3. Results
3.1. Water Quality
3.2. Growth Performance
3.3. Leukogram and Serum Biochemical Findings
3.4. Hepatic Lipid Peroxidation and Antioxidant Biomarkers
3.5. Histopathological Observations
3.6. mRNA Expression Profile
3.6.1. Relative Gene Expression of Appetite- and Growth-Related Genes
3.6.2. Relative Gene Expression of Some Glucose and Lipid Regulatory Genes
3.6.3. Relative Gene Expression of Some Inflammatory Cytokine Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| FBW | Final body weight |
| FCR | Feed conversion ratio |
| SGR | Specific growth rate |
| PER | Protein efficiency ratio |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| DO | Dissolved oxygen |
| EDTA | Ethylenediaminetetraacetic acid |
| FAS | Fatty acid synthase |
| HSPs | Heat shock proteins |
| IGF | Insulin-like growth factor |
| ILs | Interlukins |
| LDH | Lactate dehydrogenase |
| LDL-C | Low-density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| MMC | Melanomacrophage centers |
| MSTN | Myostatin gene |
| NPY | Neuropeptide Y |
| NRC | Nutrition Research Council |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PK | Pyruvate kinase |
| ROS | Reactive oxygen species |
| TAN | The total ammonia nitrogen was measured |
| TNFα | Tumor necrosis factor alpha |
| VLDL-C | Very-low-density lipoprotein cholesterol |
| YSE | Yucca schidigera extract |
References
- Yoo, G.; Bai, S.C. Effects of the dietary microbial phytase supplementation on bioavailability of phosphorus in juvenile olive flounder Paralichthys olivaceus fed soybean meal based diets. Fish. Aquatic Sci. 2014, 17, 319–324. [Google Scholar] [CrossRef] [Green Version]
- The State of World Fisheries and Aquaculture. 2014. Available online: http://www.fao.org/resources/infographics/infographics-details/en/c/231544/ (accessed on 14 December 2020).
- Boyd, C.E.; Torrans, E.L.; Tucker, C.S. Dissolved oxygen and aeration in Ictalurid catfish aquaculture. J. World Aquac. Soc. 2018, 49, 7–70. [Google Scholar] [CrossRef]
- De Leão, S.R.; Zaniboni-Filho, E.; Baldisserotto, B. Effect of combined non-ionized ammonia and dissolved oxygen levels on the survival of juvenile dourado, Salminus brasiliensis (Cuvier). J. World Aquac. Soc. 2009, 40, 695–701. [Google Scholar] [CrossRef]
- El-Shafai, S.A.; El-Gohary, F.A.; Nasr, F.A.; van der Steen, N.P.; Gijzen, H.J. Chronic ammonia toxicity to duckweed-fed tilapia (Oreochromis niloticus). Aquaculture 2004, 232, 117–127. [Google Scholar] [CrossRef]
- Sinha, A.L.; Liew, H.J.; Diricxa, M.; Blust, R.; Boeck, G.D. The interactive effectsof ammonia exposure, nutritional status and exercise on metabolic and physiological responses in gold fish (Carassius auratus L.). Aquat. Toxicol. 2012, 109, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wajsbrot, N.; Gasith, A.; Diamant, A.; Popper, D.M. Chronic toxicity of ammonia to juvenile gilthead seabream Sparus aurata and related histopathological effects. J. Fish. Biol. 1993, 42, 321–328. [Google Scholar] [CrossRef]
- Thurston, R.V.; Philipps, G.R.; Russo, R.C. Increased toxicity of ammonia to rainbow trout (S. gairdneri) resulting from reduced concentrations of dissolved oxygen. Can. J. Fish. Aquat. Sci. 1981, 38, 983–988. [Google Scholar] [CrossRef]
- Lemarie, G.; Dosdat, A.; Coves, D.; Dutto, G.; Gasset, E.; Person-Le Ruyet, J. Effect of chronic ammonia exposure on growth of European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2004, 229, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Benli, A.C.K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia(Oreochromis niloticus L.): Effects on gill liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef]
- Murthy, C.R.K.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neuro. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine, 2nd ed.; Oxford Clarendon Press: England, UK, 1989; pp. 22–85. [Google Scholar]
- Nirupama, R.; Rajaraman, B.; Yajurvedi, H.N. Stress and Glucose metabolism: A Review. Imaging J. Clin. Med Sci. 2018, 5, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Schram, E.; Verdegem, M.C.J.; Widjaja, R.T.O.B.H.; Kloet, C.J.; Foss, A.; Schelvis-Smit, R.; Roth, B.; Imsland, A.K. Impact of increased flow rate on specific growth rate of juvenile turbot (Scophthalmus maximus, Rafinesque 1810). Aquaculture 2009, 292, 46–52. [Google Scholar] [CrossRef]
- Small, B.C.; Davis, K.B.; Peterson, B.C. Elucidating the effects of cortisol and stress on economically important traits in channel catfish. N. Am. J. Aquac. 2008, 70, 223–235. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1, 8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Rajabiesterabadim, H.; Yousefi, M.; Hoseini, S.M. Enhanced haematological and immune responses in common carp Cyprinus carpio fed with olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquac. Nut. 2020, 26, 763–771. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Varma, A. Medicinal Plants and Environmental Challenges; Springer Publishing: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; Oleszek, W. Saponins and Phenolics of Yucca schidigera Roezl: Chemistry and Bioactivity. Phytochem. Rev. 2005, 4, 177–190. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; El-Kholy, M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2016, 23, 6774–6782. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Karimi, M.; Raieni, R.F.; Dadar, M.; Yilmaz, S.; Dawood, A.A.O. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An Overview. Rev. Fish. Sci. Aqua. 2020. [Google Scholar] [CrossRef]
- Farag, R.M.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Samak, D.H. Yucca schidigera extract modulates the lead-induced oxidative damage, nephropathy and altered inflammatory response and glucose homeostasis in Japanese quails. Ecotox Environ. Saf. 2018, 156, 311–321. [Google Scholar] [CrossRef]
- Ashour, E.A.; Alagawany, M.; Reda, F.M.; Abd El-Hac, M.E. Effect of supplementation of Yucca schidigera extract to growing rabbit diets on growth performance, carcass characteristics, serum biochemistry and liver oxidative status. Asian J. Anim. Vet. Adv. 2014, 9, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Kucukkurt, I.; Akkol, E.K.; Karabag, F.; Ince, S.; Süntar, I.; Eryavuz, A.; Sözbilir, N.B. Determination of the regulatory properties of Yucca schidigera extracts on the biochemical parameters and plasma hormone levels associated with obesity. Rev. Bras. Farmacogn. 2016, 26, 246–250. [Google Scholar] [CrossRef]
- Deng, J.M.; Mai, K.S.; Ai, Q.H.; Zhang, W.B.; Tan, B.P.; Xu, W.; Liufu, Z.G. Alternative protein sources in diets for Japanese flounder Paralichthys olivaceus (Temminck and Schlegel): II. Effects on nutrient digestibility and digestive enzyme activity. Aquac. Res. 2010, 41, 861–870. [Google Scholar] [CrossRef]
- Guyon, R.; Rakotomanga, M.; Azzouzi, N.; Coutanceau, J.P.; Bonillo, C.; D’Cotta, H.; Pepey, E.; Soler, L.; Rodier-Goud, M.; D’Honte, A.; et al. A high-resolution map of the Nile tilapia genome: A resource for studying cichlids and other percomorphs. BMC Genom. 2012, 13, 222. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Feldman, B.; Zinkl, J.; Jain, N. Schalm’s Veterinary Hematology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Friedwald, W.T.; Levy, R.I.; Fredriekson, D.S. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Kaneko, J.J. Clinical Biochemistry of Domestic Animals, 4th ed.; Academic Press lnc.: New York, NY, USA, 1989; pp. 146–159. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzyme function for erythrocuprein (hemocurrein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Owens, C.; Belcher, R. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965, 94, 705–711. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The Hematoxylin and eosin. In Theory Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone of ElSevier: Philadelphia, PA, USA, 2013; pp. 179–220. [Google Scholar]
- Yang, C.G.; Wang, X.L.; Tian, J.; Liu, W.; Wu, F.; Jiang, M.; Wen, H. Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus). Gene 2013, 527, 183–192. [Google Scholar] [CrossRef]
- Qiang, J.; Yang, H.; He, J.; Wang, H.; Zhu, Z.X.; Xu, P. Comparative Study of the Effects of Two High-Carbohydrate Diets on Growth and Hepatic Carbohydrate Metabolic Enzyme Responses in juvenile GIFT tilapia (Oreochromis niloticus). Turk. J. Fish. Aquat. Sci. 2014, 14, 515–525. [Google Scholar] [CrossRef]
- Mahfouz, E.M.; Hegazi, M.M.; El-Magd, M.A.; Kasem, E.A. Metabolic and molecular responses in Nile tilapia, Oreochromis niloticus during short and prolonged hypoxia. Mar. Fresh Behav. Physiol. 2015, 48, 319–340. [Google Scholar] [CrossRef]
- Costa, L.S.; Rosa, P.V.; Fortes-Silva, R.; S’anchez-V’azquez, F.J.; L’opez-Olmeda, J.F. Daily rhythms of the expression of genes from the somatotropic axis: The influence on tilapia (Oreochromis niloticus) of feeding and growth hormone administration at different times. Comp. Biochem. Physiol. Part. C 2015, 181–182, 27–34. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Xu, P.; Michael Habte-Tsion, H.; Ma, X.Y.; Zhu, Z.X. The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquacult. Int. 2016, 24, 1365–1378. [Google Scholar] [CrossRef]
- Tian, J.; Wu, F.; Yang, C.G.; Jiang, M.; Liu, W.; Wen, H. Dietary lipid levels impact lipoprotein lipase, hormone-sensitive lipase, and fatty acid synthetase gene expression in three tissues of adult GIFT strain of Nile tilapia, Oreochromis niloticus. Fish. Physiol. Biochem. 2014, 41, 1–18. [Google Scholar] [CrossRef]
- Yan, P.; Jia, J.; Yang, G.; Wang, D.; Sun, C.; Li, W. Duplication of neuropeptide Y and peptide YY in Nile tilapia Oreochromis niloticus and their roles in food intake regulation. Peptides 2017, 88, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Elkatatny, N.A.; Elbialy, Z.I.; El-Nahas, A.F.; Mahmoud, S. Characterization of Myostatin Gene in Nile Tilapia (Oreochromis niloticus), the Possible Association of BsmI-exon 2 Polymorphism with Its Growth. Am. J. Life Sci. 2016, 4, 82–86. [Google Scholar] [CrossRef]
- Pffafi, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, 9. [Google Scholar]
- Sun, Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free. Radic. Biol. Med. 1990, 8, 583–599. [Google Scholar] [CrossRef]
- Fayed, W.M.A.; Khalil, R.H.; Sallam, G.R.; Mansour, A.T.; Elkhayat, B.K.; Omar, E.A. Estimating the effective level of Yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and Water quality of European seabass juveniles (Dicentrarchus labrax). Aquac. Rep. 2019, 15. [Google Scholar] [CrossRef]
- Yang, S.; Du, J.; Duan, Y.L.; Xiao, Q.; Li, N.Q.; Lin, Q.; Zhao, L.L.; Du, Z.J.; Zhou, J.; Du, J. Differences in the digestive enzyme activity, intestinal mucosa and microbial community in loach cultivated in two separate environments. BMC Microbiol. 2018, 18, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.H. Study on the effects of environmental stress on the immune and. digestive physiology of Charybdis japonica. Chin. Uni. Min. Tech. 2014, 09, 12–17. [Google Scholar]
- Stern, R.A.; Dasarathy, S.; Mozdziak, P.E. Ammonia elicits a different myogenic response in avian and murine myotubes. In Vitro Cell Dev. Biol. Anim. 2014, 53, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Hatzoglou, M. Hyperammonemia and Proteostasis in Cirrhosis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 30–36. [Google Scholar] [CrossRef]
- Kang, J.D.; Kim, S.; Zhu, H.Y.; Jin, L.; Guo, Q.; Li, X.C. Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering. RSC Adv. 2017, 7, 12541–12549. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Momani, J.; Kim, Y.M.; Kang, C.K.; Choi, J.H.; Baek, H.J. Effective RNA-silencing strategy of Lv-MSTN/GDF11 gene and its effects on the growth in shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 179, 9–16. [Google Scholar] [CrossRef]
- Lv, Q.; Yuan, L.; Deng, J.; Chen, M.; Wang, Y.; Zeng, J. Efficient generation of myostatin gene mutated rabbit by CRISPR/Cas9. Sci. Rep. 2016, 6, 25029. [Google Scholar] [CrossRef] [Green Version]
- Polakof, S.; Moon, T.W.; Aguirre, P.; Skiba-Cassy, S.; Panserat, S. Glucose homeostasis in rainbow trout fed a high-carbohydrate diet: Metformin and insulin interact in a tissue-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R166–R174. [Google Scholar] [CrossRef] [Green Version]
- Librán-Pérez, M.; Polakof, S.; López-Patiño, M.A.; Míguez, J.M.; Soengas, J.L. Evidence of a metabolic fatty acid-sensing system in the hypothalamus and Brockmann bodies of rainbow trout: Implications in food intake regulation. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R1340–R1350. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.C.; Small, B.C. Effects of exogenous cortisol on the GH/IGF-I/IGFBP network in channel catfish. Domest. Anim. Endocrinol. 2005, 28, 391–404. [Google Scholar] [CrossRef]
- Gregory, T.R.; Wood, C.M. The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol. Biochem. Zool. 1999, 72, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Leal, E.; Fernández-Durán, B.; Guillot, R.; Ríos, D.; Cerdá-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Phys. B. 2011, 181, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Small, B.C.; Murdock, C.A.; Waldbieser, G.C.; Peterson, B.C. Reduction in channel catfish hepatic growth hormone receptor expression in response to food deprivation and exogenous cortisol. Domest. Anim. Endocrinol. 2006, 31, 340–356. [Google Scholar] [CrossRef]
- Chrenkova, M.; Chrastinova, P.M.; Formelova, Z.; Balaži, A.; Ondruška, L.; Sirotkin, A.V.; Chrenek, P. The effect of Yucca schidigera extract in diet of rabbits on nutrient digestibility and qualitative parameters in caecum. Slovak J. Anim. Sci. 2012, 45, 83–88. [Google Scholar]
- Aazami, M.H.; Tahmasbi, A.M.; Ghaffari, M.H.; Naserian, A.A.; Valizadeh, R.; Ghaffari, A.H. Effects of saponins on rumen fermentation, nutrients digestibility, performance, and plasma metabolites in sheep and goat kids. Annu. Res. Rev. Biol. 2013, 3, 596–607. [Google Scholar]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 29, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, D.; Fan, Z.; Li, H.; Li, J.; Zhang, Y.; Xu, Q.; Wang, G.; Zhu, Z. Effect of Yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio). Fish. Shellfish. Immunol. 2020, 103, 211–219. [Google Scholar] [CrossRef]
- Gaber, M.M. The effects of plant-protein-based diets supplemented with yucca on growth, digestibility, and chemical composition of Nile tilapia (Oreochromis niloticus, L) fingerlings. J. World Aquacult. Soc. 2006, 37, 74–81. [Google Scholar] [CrossRef]
- Njagi, G.W.; Lee, S.; Won, S.; Hong, J.; Hamidoghli, A.; Bai, S.C. Effects of dietary Yucca meal on growth, haematology, non-specific immune responses and disease resistance of juvenile Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Aqua. Res. 2017, 48, 4399–4408. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Elghandour, M.M.M.Y.; Monroy, J.C.; Abegunde, T.O.; Salem, A.Z.M.; Barbabosa-Pliego, A.; Faniyi, T.O. Potential influence of Yucca extract as feed additive on greenhouse gases emission for a cleaner livestock and aquaculture farming—A review. J. Clean. Prod. 2019, 239, 118074. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total. Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Elbialy, Z.I.; Rizk, M.; Al-Hawary, I.I.; Salah, A.S.; Mohammed, R.A.; Assar, D.H.; Almeer, R.; Dawood, M.A.O. Yucca schidigera extract mediated the growth performance, hepato-renal function, antioxidative status and histopathological alterations in Nile tilapia (Oreochromis niloticus) exposed to hypoxia stress. Aquac. Res. 2020, 52, 1965–1976. [Google Scholar] [CrossRef]
- Grzelak, A.K.; Davis, D.J.; Caraker, S.M.; Crim, M.J.; Spitsbergen, J.M.; Wiedmeyer, C.E. Stress leukogram induced by acute and chronic stress in zebrafish (Danio rerio). Comp. Med. 2017, 67, 263–269. [Google Scholar] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsk, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Jaescke, H.; Hasegawa, T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006, 26, 912–919. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.; Lee, K.H.; Lee, D.U.; Kwak, J.H.; Kim, Y.S.; Lee, S.M. Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology 2011, 282, 104–111. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Tsukamoto, H. Oxidative stress and liver disease. Prog. Liver Dis. 1996, 14, 131–159. [Google Scholar]
- Nguyen, T.T.T.; Nguyen, H.T.; Wang, P.; Chen, S. Identification and expression analysis of two pro-inflammatory cytokines, TNF-alpha and IL-8, in cobia (Rachycentron canadum L.) in response to Streptococcus dysgalactiae infection. Fish. Shellfish. Immunol. 2017, 67, 159–171. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C.; Smith, C.E. Acute toxicity of ammonia and nitrite to cutthroat trout fry. Trans. Am. Fish. Soc. 1978, 107, 361–368. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Ling, R.Z.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Gupta, S. Inhibitory potential of yucca gloriosa l. extract and isolated gloriosaol isomeric mixture on ovalbumin induced airway hyperresponsiveness in Balb/C mice. Clin. Pharmacol. Biopharm. 2014, S2, 2. [Google Scholar] [CrossRef] [Green Version]
- Alagawany, M.M.; Farag, M.R.; Dhama, K.; Abd El-Hack, M.E.; Tiwari, R.; Alam, G.M. Mechanisms and beneficial applications of resveratrol as feed additive in animal and poultry nutrition: A Review. Int. J. Pharmacol. 2015, 11, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W. Inhibition of oxidative stress in blood platelets by different phenolics from Yucca schidigera Roezl bark. Nutrition 2003, 19, 633–640. [Google Scholar] [CrossRef]
- Damon, M.; Louveau, I.; Lefaucheur, L.; Lebret, B.; Vincent, A.; Leroy, P.; Sanchez, M.P.; Herpin, P.; Gondret, F. Number of intramuscular adipocytes and fatty acid binding protein-4 content are significant indicators of intramuscular fat level in crossbred Large White × Duroc pigs. J. Anim. Sci. 2006, 84, 1083–1092. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef]
- Sa, R.N.; Xing, H.; Luan, S.J.; Sun, Y.B.; Sun, C.Y.; Zhang, H.F. Atmospheric ammonia alters lipid metabolism-related genes in the livers of broilers (Gallus gallus). J. Anim. Physiol. Anim. Nutr. 2018, 102, e941–e947. [Google Scholar] [CrossRef]
- Yi, B.; Chen, L.; Sa, R.; Zhong, R.; Xing, H.; Zhang, H. High concentrations of atmospheric ammonia induce alterations of gene expression in the breast muscle of broilers (Gallus gallus) based on RNA-Seq. BMC Genom. 2016, 17, 598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chengdong, H.; Ming, L.; Yunxia, Q.; Wenbin, X.; Fanxing, M.; Rixin, W. Modulation of lipid metabolism in juvenile yellow catfish (Pelteobagrus fulvidraco) as affected by feeding frequency and environmental ammonia. Fish. Physiol. Biochem. 2019, 45, 115–122. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Jiang, D.L.; Li, B.J.; Qin, H.; Meng, Z.N.; Lin, H.R.; Xia, J.H. Differential transcriptomic and metabolomic responses in the liver of Nile Tilapia (Oreochromis niloticus) exposed to acute ammonia. Mar. Biotechnol. 2019, 21, 488–502. [Google Scholar] [CrossRef]
- Madison, B.N.; Tavakoli, S.; Kramer, S.; Bernier, N.J. Chronic cortisol and the regulation of food intake and the endocrine growth axis in rainbow trout. J. Endocrin. 2018, 226, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish. Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Baltzegar, D.A.; Reading, B.J.; Douros, J.D.; Borski, R.J. Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J. Endocrin. 2014, 220, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilkis, S.J.; Granner, D.K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 1992, 54, 885–909. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, J.; Naderi, F.; Chivite, M.; Soengas, J.L.; Míguez, J.M.; López-Patiño, M.A. Influence of Stress on Liver Circadian Physiology. A Study in Rainbow Trout, Oncorhynchus mykiss, as Fish Model. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Nayanatara, A.K.; Nagaraja, H.S.; Ramaswamy, C.; Bhagyalakshmi, K.; Ramesh, B.M.; Gowda, K.M.D.; Mantur, V.S. Effect of chronic unpredictable stressors on some selected lipid parameters and biochemical parameters in Wistar rats. J. Chin. Clin. Med. 2009, 4, 92–97. [Google Scholar]
- Nagaraja, H.S.; Anupama, B.K.; Jaganathan, P.S. Stress response in albino rats. Thai. J. Physiol. Sci. 2006, 19, 8–15. [Google Scholar]
- Vijayan, M.M.; Reddy, P.K.; Leatherland, J.F.; Moon, T.W. The effects of cortisol on hepatocyte metabolism in rainbow trout: A study using the steroid analogue RU486. Gen. Comp. Endocrinol. 1994, 96, 75–84. [Google Scholar] [CrossRef]
- Wang, M. The role of glucocorticoids action in the pathophysiology of metabolic syndrome. Nutr. Metab. 2005, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Aluru, N.; Vijayan, M.M. Hepatic transcriptome response to glucocorticoid receptor activation in rainbow trout. Phys. Genom. 2007, 31, 483–491. [Google Scholar] [CrossRef]
- Momoda, T.S.; Schwindt, A.R.; Feist, G.W.; Gerwick, L.; Bayne, C.J.; Schreck, C.B. Gene expression in the liver of rainbow trout, Oncorhynchus mykiss, during the stress response. Comp. Biochem. Physiol. 2007, 2D, 303–315. [Google Scholar] [CrossRef]
- Marandel, L.; Dai, W.; Panserat, S.; Skiba-Cassy, S. The five glucose6-phosphatase paralogous genes are differentially regulated by insulin alone or combined with high level of amino acids and/or glucose in trout hepatocytes. Mol. Biol. Rep. 2016, 43, 207–211. [Google Scholar] [CrossRef]
- Song, X.; Marandel, L.; Dupont-Nivet, M.; Quillet, E.; Geurden, I.; Panserat, S. Hepatic glucose metabolic responses to digestible dietary carbohydrates in two isogenic lines of rainbow trout. Biol. Open 2018, 7, 6. [Google Scholar] [CrossRef] [Green Version]







| Ingredients | % | Chemical Composition | % |
|---|---|---|---|
| Fish meal (62%) | 5.80 | Crude protein (%) | 30.08 |
| Soybean meal (48%) | 38.00 | Lipid (%) | 6.30 |
| Wheat middling | 6.80 | Fibre (%) | 5.10 |
| Corn (7.5% CP) | 28.25 | Ash (%) | 6.01 |
| Corn Gluten (63.1) | 6.00 | Gross energy (K.cal/kg) | 4180.8 |
| Fish oil | 1.40 | ||
| Dicalcium Phosphate | 0.30 | ||
| Rice bran | 13.10 | ||
| Vitamin C (35%) | 0.05 | ||
| Vitamin and mineral mix | 0.30 |
| Gene | Primer Sequence 5′-3′ | NCBI Accession Number | Reference |
|---|---|---|---|
| 18s rRNA * | F: GGACACGGAAAGGATTGACAG R: GTTCGTTATCGGAATTAACCAGAC | JF698683 | [38] |
| G6Pase | F:AGCGCGAGCCTGAAGAAGTACT R:ATGGTCCACAGCAGGTCCACAT | XM_003448671 | [39] |
| PEPCK | F: GCCCTCAGTCCAGCTGTAAG R: CACATCCCTCGGGTCAGTTC | XM_003448375.4 | This study |
| PK | F:CCGTAAGGCTGCAGACGTGCA R: ATCTGCGCACGCCCTCATGG | DQ066876.1 | [40] |
| IGF1 | For: TCCTGTAGCCACACCCTCTC R: ACAGCTTTGGAAGCAGCACT | NM_001279503.1 | [41] |
| HSP70 | F: CATCGCCTACGGTCTGGACAA R: TGCCGTCTTCAATGGTCAGGAT | FJ207463.1 | [42] |
| IL1β | F: CAAGGATGACGACAAGCCAACC R: AGCGGACAGACATGAGAGTGC | XM_003460625.2 | [42] |
| TNFα | F: GGAAGCAGCTCCACTCTGATGA R: CACAGCGTGTCTCCTTCGTTCA | JF957373.1 | [42] |
| FAS | F: TGAAACTGAAGCCTTGTGTGCC R: TCCCTGTGAGCGGAGGTGATTA | GU433188.1 | [43] |
| LPL | F: TGCTAATGTGATTGTGGTGGAC R: GCTGATTTTGTGGTTGGTAAGG | GU433189.1 | [43] |
| NPYa | F: ACAAGACAGAGGTATGGGAAGA R: GGCAGCATCACCACATTG | XM_003448854.1 | [44] |
| MSTN | F: GCATCTGTCTCAGATCGTGCT R: TGCCATCATTACAATTGTCTCCG | KT987208.1 | [45] |
| Control | Ammonia | Ammonia + Yucca | Yucca | p Value | |
|---|---|---|---|---|---|
| Dissolved oxygen (mg/L) | 5.59 ± 0.41 | 5.16 ± 0.43 | 4.92 ± 0.16 | 5.64 ± 0.76 | 0.322 |
| Temperature (°C) | 26.59 ± 0.42 | 27.2 ± 0.8 | 26.9 ± 0.72 | 26.80 ± 0.59 | 0.381 |
| pH | 7.94 ± 0.21 | 8.18 ± 0.11 | 8.03 ± 0.17 | 7.81 ± 0.27 | 0.419 |
| TAN (mg/L) | 0.37 ± 0.09 c | 4.1 ± 0.03 a | 1.41 ± 0.02 b | 0.41 ± 0.12 c | 0.001 |
| UIA (mg/L) | 0.0194 ± 0.006 c | 0.44 ± 0.02 a | 0.124 ± 0.04 b | 0.016 ± 0.001 c | 0.0017 |
| Control | Ammonia | Ammonia + Yucca | Yucca | p-Value | |
|---|---|---|---|---|---|
| WBCs (×103/µL) | 16.77 ± 0.84 b,c | 23.84 ± 1.42 a | 19.65 ± 0.85 b | 18.69 ± 0.45 c | 0.0016 |
| Neutrophils (×103/µL) | 6.1 ± 0.33 c | 14.19 ± 0.94 a | 8.87 ± 0.48 b | 7.3 ± 0.84 c | 0.0001 |
| Lymphocytes (×103/µL) | 9.9 ± 1.76 b | 7.87 ± 0.317 c | 9.56 ± 0.22 b | 10.5 ± 1.61 a | 0.0117 |
| Monocytes (×103/µL) | 0.564 ± 0.05 c | 1.63 ± 0.24 a | 0.86 ± 0.119 b | 0.689 ± 0.03 c | 0.005 |
| Eosinophils (×103/µL) | 0.208 ± 0.05 a | 0.15 ± 0.03 b | 0.272 ± 0.27 a | 0.206 ± 0.02 a | 0.002 |
| ALT (U/L) | 14.33 ± 3.85 c | 64.21 ± 3.84 a | 26.67 ± 6.8 b | 14.1 ± 2.6 c | 0.0001 |
| AST (U/L) | 48.67 ± 4.49 c | 105.7 ± 8.09 a | 63.01 ± 12.06 b | 44.3 ± 6.69 c | 0.005 |
| LDH (U/L) | 457 ± 40.22 c | 914.5 ± 50.22 a | 580.4 ± 34.2 b | 487 ± 0.34 c | 0.001 |
| Total Protein (g/dL) | 4.36 ± 0.33 a | 3.08 ± 0.17 c | 4.1 ± 0.05 b | 4. 79 ± 0.15 a | 0.003 |
| Albumin (g/dL) | 2.6 ± 0.15 a | 1.7 ± 0.25 b | 2.26 ± 0.23 a | 2.5 ± 0.06 a | 0.016 |
| Globulin (g/dL) | 1.76 ± 0.19 | 1.38 ± 0.24 | 1.84 ± 2.08 | 2.29 ± 0.17 | 0.669 |
| A/G ratio | 1.48 ± 0.24 | 1.23 ± 0.34 | 1.23 ± 0.54 | 1.09 ± 0.24 | 0.4944 |
| Cholesterol (mg/dL) | 130.67 ± 11.79 a | 106.3 ± 11.7 c | 129.7 ± 28.9 b | 134.33 ± 21.17 a | 0.002 |
| Triglyceride (mg/dL) | 383 ± 56.63 a | 184 ± 38.5 c | 324 ± 24.17 b | 402 ± 187.02 a | 0.0017 |
| HDL (mg/dL) | 33 ± 5.29 a | 21.67 ± 3.28 b | 31.3 ± 3.12 a | 35.67 ± 6.94 a | 0.0017 |
| LDL (mg/dL) | 21.07 ± 0.86 c | 47.83 ± 16.48 a | 33.6 ± 27.74 b | 18.26 ± 0.77 c | 0.0058 |
| VLDL (mg/dL) | 76.6 ± 2.28 a | 36.8 ± 1.10 c | 64.8 ± 1.92 b | 80.4 ± 3.50 a | 0.002 |
| Amylase (U/L) | 70.5 ± 1.5 a | 65 ± 20 b | 75.5 ± 9.5 a | 73.1 ± 7.03 a | 0.005 |
| Lipase (U/L) | 35.5 ± 2.51 a | 18.2 ± 2.1 b | 30.67 ± 0.88 a | 30.1 ± 2.12 a | 0.005 |
| Urea | 14.01 ± 1.53 b | 42.03 ± 10.79 a | 20.32 ± 1.20 b | 16.67 ± 1.85 b | 0.0114 |
| Glucose (mg/dL) | 74.33 ± 2.3 c | 298.3 ± 41.2 a | 96.3 ± 5.77 b | 82.1 ± 3.09 c | 0.0015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbialy, Z.I.; Salah, A.S.; Elsheshtawy, A.; Rizk, M.; Abualreesh, M.H.; Abdel-Daim, M.M.; Salem, S.M.R.; Askary, A.E.; Assar, D.H. Exploring the Multimodal Role of Yucca schidigera Extract in Protection against Chronic Ammonia Exposure Targeting: Growth, Metabolic, Stress and Inflammatory Responses in Nile Tilapia (Oreochromis niloticus L.). Animals 2021, 11, 2072. https://doi.org/10.3390/ani11072072
Elbialy ZI, Salah AS, Elsheshtawy A, Rizk M, Abualreesh MH, Abdel-Daim MM, Salem SMR, Askary AE, Assar DH. Exploring the Multimodal Role of Yucca schidigera Extract in Protection against Chronic Ammonia Exposure Targeting: Growth, Metabolic, Stress and Inflammatory Responses in Nile Tilapia (Oreochromis niloticus L.). Animals. 2021; 11(7):2072. https://doi.org/10.3390/ani11072072
Chicago/Turabian StyleElbialy, Zizy I., Abdallah S. Salah, Ahmed Elsheshtawy, Merna Rizk, Muyassar H. Abualreesh, Mohamed M. Abdel-Daim, Shimaa M. R. Salem, Ahmad El Askary, and Doaa H. Assar. 2021. "Exploring the Multimodal Role of Yucca schidigera Extract in Protection against Chronic Ammonia Exposure Targeting: Growth, Metabolic, Stress and Inflammatory Responses in Nile Tilapia (Oreochromis niloticus L.)" Animals 11, no. 7: 2072. https://doi.org/10.3390/ani11072072