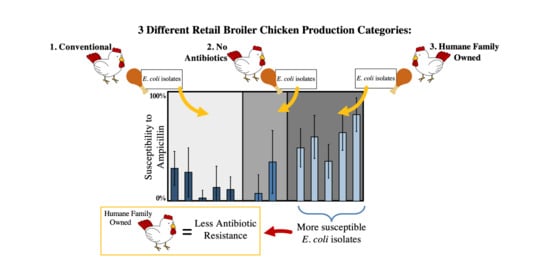
Antibiotic Resistance of Escherichia coli Isolated from Conventional, No Antibiotics, and Humane Family Owned Retail Broiler Chicken Meat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assigning Production Categories to Retail Chicken Parts
2.2. Meat Selection and Bacterial Purification
2.3. Antibiotic Resistance Testing: Kirby-Bauer Disk Diffusion Susceptibility Test
2.4. Statistics and Data Analysis
3. Results
3.1. Fraction of Resistant, Intermediate, and Susceptibility Isolates from Each Category of Chicken
3.2. Statistical Differences in Antimicrobial Resistance in Chicken Production Groupings.
4. Discussion
4.1. Environmental and Human Health Relevance of Erythromycin Resistance in Gram Negative Isolates
4.2. Significantly FewerAntibiotic-Resistant Isolates in Humane Family Owned Chicken
4.3. Support for the Prioritization of One Health Standards to Combat Antimicrobial Resistance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT: Live Animals Data. Available online: http://www.fao.org/faostat/en/#data/QA/visualize (accessed on 4 November 2020).
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- The Review on Antimicrobial Resistance. Tackling Drug Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Wang, Y.; Sun, Y.; Ma, L.; Zeng, Q.; Jiang, X.; Li, A.; Zeng, Z.; Zhang, T. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, J.; Brisbin, J.T.; Yu, H.; Sanei, B.; Sabour, P.; Sharif, S. Appropriate chicken sample size for identifying the composition of broiler intestinal microbiota affected by dietary antibiotics, using the polymerase chain reaction-denaturing gradient gel electrophoresis technique. Poult. Sci. 2007, 86, 2541–2549. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Kumar, K.C.; Gupta, S.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar]
- Luangtongkum, T.; Morishita, T.Y.; Ison, A.J.; Huang, S.; McDermott, P.F.; Zhang, Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol. 2006, 72, 3600–3607. [Google Scholar] [CrossRef] [Green Version]
- Swann Committee. Report of Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine; Her Majesty’s Stationary Office: London, UK, 1969. [Google Scholar]
- WHO. WHO|WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Expert Commission on Addressing the Contribution of Livestock to the Antibiotic Resistance Crisis. COMBATING ANTIBIOTIC RESISTANCE A Policy Roadmap to Reduce Use of Medically Important Antibiotics in Livestock; Expert Commission on Addressing the Contribution of Livestock to the Antibiotic Resistance Crisis: Washington, DC, USA, 2017. [Google Scholar]
- CDC. Antibiotic Resistance Threats in the United States; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- The Pew Charitable Trusts Antibiotics and Animal Agriculture: A Primer|The Pew Charitable Trusts. Available online: https://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2016/12/antibiotics-and-animal-agriculture-a-primer (accessed on 25 October 2020).
- U.S. Food and Drug Administration. 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/media/133411/download (accessed on 16 November 2020).
- Levy, S.B.; Fitzgerald, G.B.; Macone, A.B. Changes in Intestinal Flora of Farm Personnel after Introduction of a Tetracycline-Supplemented Feed on a Farm. N. Engl. J. Med. 1976, 295, 583–588. [Google Scholar] [CrossRef]
- Levy, S.B. Emergence of Antibiotic-Resistant Bacteria in the Intestinal Flora of Farm Inhabitants. J. Infect. Dis. 1978, 137, 688–690. [Google Scholar] [CrossRef]
- van den Bogaard, A.E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother 2001, 47, 763–771. [Google Scholar] [CrossRef]
- Levy, S.B.; Bonnie, M. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Price, L.B.; Lackey, L.G.; Vailes, R.; Silbergeld, E. The persistence of fluoroquinolone-resistant Campylobacter in poultry production. Environ. Health Perspect. 2007, 115, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- WHO. Antimicrobial Resistance Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- The Interagency Task Force for Combating Antibiotic Resistance. National Action Plan for Combating Antibiotic-Resistant Bacteria; The White House: Washington, DC, USA, 2015.
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef]
- Hawthorn, A.; Kleidon, T.; Larsen, E.; Marsh, N.; Marshall, A.; Ray-Barruel, G.; Sinclair, S.; Slaughter, E.; St John, A.; Taliaferro, K.; et al. Peripheral Intravenous Catheter Protection. Br. J. Nurs. 2018, 30, 28. [Google Scholar]
- Bergeron, C.R.; Prussing, C.; Boerlin, P.; Daignault, D.; Dutil, L.; Reid-Smith, R.J.; Zhanel, G.G.; Manges, A.R. Chicken as reservoir for extraintestinal pathogenic Escherichia coli in Humans, Canada. Emerg. Infect. Dis. 2012, 18, 415–421. [Google Scholar] [CrossRef]
- Nordstrom, L.; Liu, C.M.; Price, L.B. Foodborne urinary tract infections: A new paradigm for antimicrobial-resistant foodborne illness. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Porter, S.B.; Johnston, B.; Thuras, P.; Clock, S.; Crupain, M.; Rangan, U. Extraintestinal pathogenic and antimicrobialresistant Escherichia coli, including sequence type 131 (ST131), from retail chicken breasts in the United States in 2013. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Campagnolo, E.R.; Johnson, K.R.; Karpati, A.; Rubin, C.S.; Kolpin, D.W.; Meyer, M.T.; Esteban, J.E.; Currier, R.W.; Smith, K.; Thu, K.M.; et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002, 299, 89–95. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture USDA Process Verified Program: Transparency from Farm to Market|USDA. Available online: https://www.usda.gov/media/blog/2015/12/07/usda-process-verified-program-transparency-farm-market (accessed on 16 November 2020).
- Chicken Standards & Application—Global Animal Partnership. Available online: https://globalanimalpartnership.org/standards/chicken/ (accessed on 29 March 2020).
- Consumer Reports Consumer Reports Survey Show 73 Percent of Consumers Look for ‘Natural’ Labels at Grocery Stores—and Many Are Unwittingly Misled. Available online: https://www.consumerreports.org/media-room/press-releases/2016/05/consumer-reports-survey-show-73-percent-of-consumers-misled-by-natural-labels-at-the-grocery-store/ (accessed on 25 October 2020).
- McDonald’s Global Vision for Antibiotic Stewardship in Food Animals. 2017. Available online: https://www.comunicarseweb.com/sites/default/files/mcdonalds-global-vision-for-antimicrobial-stewardship-in-food.pdf (accessed on 26 October 2020).
- Millman, J.M.; Waits, K.; Grande, H.; Marks, A.R.; Marks, J.C.; Price, L.B.; Hungate, B.A. Prevalence of antibiotic-resistant E. coli in retail chicken: Comparing conventional, organic, kosher, and raised without antibiotics. F1000Research 2013, 2, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, C.J. Agroecosystem Management and Nutritional Quality of Plant Foods: The Case of Organic Fruits and Vegetables. CRC. Crit. Rev. Plant Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Miranda, J.M.; Vázquez-Belda, B.; Fente, C.A.; Calo-Mata, P.; Cepeda, A.; Franco, C.M. Comparison of Antimicrobial Resistance in Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes Strains Isolated from Organic and Conventional Poultry Meat. J. Food Prot. 2008, 71, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.R.; Hulet, R.M.; Zhang, G.; McDermott, P.; Kinney, E.L.; Schwab, K.J.; Joseph, S.W. Lower prevalence of antibiotic-resistant enterococci on U.S. conventional poultry farms that transitioned to organic practices. Environ. Health Perspect. 2011, 119, 1622–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, K.; Lai, J.; Wu, C.; Shen, J.; Wang, Y. Prevalence and antimicrobial resistance of Enterococcus species of food animal origin from Beijing and Shandong Province, China. J. Appl. Microbiol. 2013, 114, 555–563. [Google Scholar] [CrossRef]
- Obeng, A.S.; Rickard, H.; Ndi, O.; Sexton, M.; Barton, M. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Vet. Microbiol. 2012, 154, 305–315. [Google Scholar] [CrossRef] [PubMed]
- CDC National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS)|NARMS|CDC. Available online: https://www.cdc.gov/narms/index.html (accessed on 15 July 2020).
- Global Animal Partnership. Global Animal Partnership’s 5-Step TM Animal Welfare Rating Standards for Chickens Raised for Meat v3.1; Global Animal Partnership: Austin, TX, USA, 2018; pp. 1–33. [Google Scholar]
- Humane Farm Animal Care. Humane Farm Animal Care Program Policy Manual; Humane Farm Animal Care: Middleburg, VA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; ISBN 978-1-68440-067-6. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Bower, K.M. When to use Fisher’s Exact Test. Am. Soc. Qual. 2003, 2, 35–37. [Google Scholar]
- McCrum-Gardner, E. Which is the correct statistical test to use? Br. J. Oral Maxillofac. Surg. 2008, 46, 38–41. [Google Scholar] [CrossRef]
- Zhang, J.; Massow, A.; Stanley, M.; Papariella, M.; Chen, X.; Kraft, B.; Ebner, P. Contamination Rates and Antimicrobial Resistance in Enterococcus spp., Escherichia coli, and Salmonella Isolated from “No Antibiotics Added”–Labeled Chicken Products. Foodborne Pathog. Dis. 2011, 8, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Akond, M.A.; Hassan, S.M.R.; Slam, S.; Shirin, M. Antibiotic Resistance of Escherichia Coli Isolated from Poultry and Poultry Environment of Bangladesh. Am. J. Environ. Sci. 2009, 5, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Kibret, M.; Abera, B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr. Health Sci. 2011, 11, S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, M.; Courvalin, P. Contribution of two different mechanisms to erythromycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 1986, 30, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [Green Version]
- McEwen, S.A. Human Health Importance of use of Antimicrobials in Animals and Its Selection of Antimicrobial Resistance. In Antimicrobial Resistance in the Environment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 389–422. [Google Scholar]
- Travers, K.; Barza, M. Morbidity of Infections Caused by Antimicrobial-Resistant Bacteria. Clin. Infect. Dis. 2002, 34, S131–S134. [Google Scholar] [CrossRef] [Green Version]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Lipsitch, M.; Singer, R.S.; Levin, B.R. Antibiotics in agriculture: When is it time to close the barn door? Proc. Natl. Acad. Sci. USA 2002, 99, 5752–5754. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef]
- Matte-Tailliez, O.; Brochier, C.; Forterre, P.; Philippe, H. Archaeal Phylogeny Based on Ribosomal Proteins. Mol. Biol. Evol. 2002, 19, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedenbeck, J.; Cohan, F.M. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev. 2011, 35, 957–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleha, A.A.; Tin, T.M.; Ganapathy, K.K.; Zulkifli, I.; Raha, R.; Arifah, K. Possible effect of antibiotic-supplemented feed and environment on the occurrence of multiple antibiotic resistant Escherichia coli in chickens. Int. J. Poult. Sci. 2009, 8, 28–31. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Marketing Service. Organic Livestock Requirements; United States Department of Agriculture: Washington, DC, USA, 2013.


| Certified Humane | GAP Level 3+ | Organic | No Antibiotics Ever | Raised without Antibiotics | Free Range | All Natural | Conventional | |
|---|---|---|---|---|---|---|---|---|
| Antibiotic Use 3 | Only when sick and under veterinarian care After treatment, the label may still be used 4,5 | Only when sick and under veterinarian care After treatment, the label may not be used (5) 6 | May be injected in ovo 7 and used on first day of life Prohibited after first day of life Sick animals may be treated with antibiotics under veterinary supervision but the label may not be used 8 | Only when sick and under veterinarian care After treatment, the label may not be used (5) | May be injected in ovo Prohibited after birth Sick animals may be treated with antibiotics under veterinary supervision but the label may not be used 9 | No Requirement | No Requirement | No Requirement |
| Outdoor Access | No Requirement 10 | Outdoor access must be at least 25% of indoor area Outdoor access required beginning at four weeks of life (6) | Specific amount of required outdoor space is not given 11,12 Outdoor access must be available for at least 50% of life. | No Requirement | No Requirement | No Requirement | No Requirement | No Requirement |
| Animal Byproducts in Feed | Allowed | Prohibited | Prohibited | Allowed | Allowed | Allowed | Allowed | Allowed |
| Space Requirements | 1 sq. ft per 6 lbs of animal (4) | 1 sq. ft per 6.5 lbs of animal (4) | No Requirement | No Requirement | No Requirement | No Requirement | No Requirement | No Requirement |
| GMOs in Feed | Allowed | Allowed | Prohibited | Allowed | Allowed | Allowed | Allowed | Allowed |
| Light Requirement | Six hours of continuous darkness per day after first day of life (4) | Eight hours of continuous darkness per day after first day of life (6) | No Requirement | No Requirement | No Requirement | No Requirement | No Requirement | No Requirement |
| Slaughter Requirements | Birds must be stunned and insensible to pain Slaughter facilities undergo annual audits (4) | Only standard USDA slaughter requirements 13 | Only standard USDA slaughter requirements | One-time desk audit to ensure adequate segregation between animals given antibiotics and those that were not 14 | One-time desk audit to ensure adequate segregation between animals given antibiotics and those that were not 15 | Only standard USDA slaughter requirements | Only standard USDA slaughter requirements | Only standard USDA slaughter requirements |
| Audit by a Third Party | n/a—They are a third party that performs farm audits | n/a—They are a third party that performs farm audits | Yes 16 | None | None | None | None | None |
| Clearly Published Standards for Label Use | Yes (4) | Yes (6) | Yes 17 | No, farmers submit documentation to the USDA to apply for this label (16) | No, farmers submit documentation to the USDA to apply for this label (9) | No, farmers submit documentation to the USDA to apply for this label 18 | Yes | n/a |
| Certified Humane | GAP Level 3+ | Organic | No Antibiotics Ever | Raised without Antibiotics | Free Range | All Natural | ||
|---|---|---|---|---|---|---|---|---|
| Conventional | C-1 | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ |
| C-2 | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ | |
| C-3 | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ | |
| C-4 | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ | |
| C-5 | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ | |
| No Anti-biotics | NA-1 | ☐ | ☐ | ☐ | ■ | ☐ | ☐ | ■ |
| NA-2 | ☐ | ☐ | ■ | ☐ | ■ | ■ | ■ | |
| Humane Family Owned | HFO-1 | ☐ | ■ | ■ | ☐ | ☐ | ■ | ☐ |
| HFO-2 | ☐ | ■ | ☐ | ■ | ☐ | ☐ | ☐ | |
| HFO-3 | ☐ | ■ | ☐ | ■ | ☐ | ☐ | ☐ | |
| HFO-4 | ■ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ | |
| HFO-5 | ■ | ☐ | ☐ | ☐ | ☐ | ☐ | ■ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, H.M.; Whitener, V.A.; Thulsiraj, V.; Amundson, A.; Collins, C.; Duran-Gonzalez, M.; Giragossian, E.; Hornstra, A.; Kamel, S.; Maben, A.; et al. Antibiotic Resistance of Escherichia coli Isolated from Conventional, No Antibiotics, and Humane Family Owned Retail Broiler Chicken Meat. Animals 2020, 10, 2217. https://doi.org/10.3390/ani10122217
Sanchez HM, Whitener VA, Thulsiraj V, Amundson A, Collins C, Duran-Gonzalez M, Giragossian E, Hornstra A, Kamel S, Maben A, et al. Antibiotic Resistance of Escherichia coli Isolated from Conventional, No Antibiotics, and Humane Family Owned Retail Broiler Chicken Meat. Animals. 2020; 10(12):2217. https://doi.org/10.3390/ani10122217
Chicago/Turabian StyleSanchez, Helen M., Victoria A. Whitener, Vanessa Thulsiraj, Alicia Amundson, Carolyn Collins, Mckenzie Duran-Gonzalez, Edwin Giragossian, Allison Hornstra, Sarah Kamel, Andrea Maben, and et al. 2020. "Antibiotic Resistance of Escherichia coli Isolated from Conventional, No Antibiotics, and Humane Family Owned Retail Broiler Chicken Meat" Animals 10, no. 12: 2217. https://doi.org/10.3390/ani10122217