Latin American Origin Is Not Associated with Worse Outcomes among Hospitalized Patients with COVID-19 in a Public Healthcare System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Variables and Data Collection
2.2. Outcomes
- All-cause mortality (either in hospital or after discharge) and associated factors.
- Invasive mechanical ventilation requirement and associated factors.
- Secondary outcomes: The need for non-invasive ventilator support and ICU admission.
2.3. Statistics
3. Results
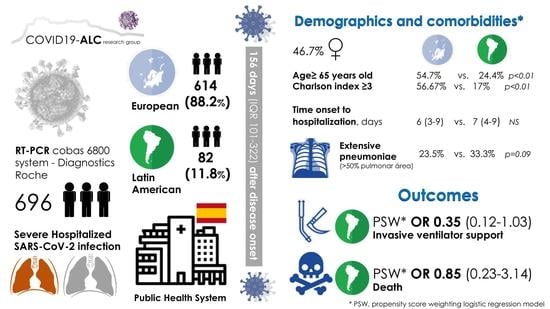
3.1. Demographics and Comorbidities
3.2. Clinical Presentation, Initial Assessment, and Treatment
3.3. Main Outcomes and Associated Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulson, M.; Neufeld, M.; Geary, A.; Kenzik, K.; Sanchez, S.E.; Dechert, T.; Kimball, S. Intersectional Disparities Among Hispanic Groups in COVID-19 Outcomes. J. Immigr. Minor. Health 2021, 23, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Telle, K.E.; Grøsland, M.; Helgeland, J.; Håberg, S.E. Factors associated with hospitalization, invasive mechanical ventilation treatment and death among all confirmed COVID-19 cases in Norway: Prospective cohort study. Scand. J. Public Health 2021, 49, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, L.; Martin, M.; Frank, M.G.; Farfan, J.F.; Kearns, M.; Rubio, L.A.; Tong, A.; Matus Gonzalez, A.; Camacho, C.; Collings, A.; et al. Experiences of Latinx Individuals Hospitalized for COVID-19: A Qualitative Study. JAMA Netw. Open 2021, 4, e210684. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.L.; Kornilov, S.A.; Roper, R.T.; Cohen-Cline, H.; Jade, K.; Smith, B.; Heath, J.R.; Diaz, G.; Goldman, J.D.; Magis, A.T.; et al. Characteristics and Factors Associated with COVID-19 Infection, Hospitalization, and Mortality Across Race and Ethnicity. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- COVID-19 Provisional Counts—Health Disparities. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid19/health_disparities.htm (accessed on 23 March 2021).
- Distribution of COVID-19 Deaths and Populations, by Jurisdiction, Age, and Race and Hispanic Origin Data Centers for Disease Control and Prevention. Available online: https://data.cdc.gov/NCHS/Distribution-of-COVID-19-Deaths-and-Populations-by/jwta-jxbg (accessed on 21 July 2021).
- Podewils, L.J.; Burket, T.L.; Mettenbrink, C.; Steiner, A.; Seidel, A.; Scott, K.; Cervantes, L.; Hasnain-Wynia, R. Disproportionate Incidence of COVID-19 Infection, Hospitalizations, and Deaths Among Persons Identifying as Hispanic or Latino—Denver, Colorado March-October 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Racial Data Transparency. Available online: https://coronavirus.jhu.edu/data/racial-data-transparency (accessed on 21 July 2021).
- Norman, F.F.; Crespillo-Andújar, C.; Pérez-Molina, J.A.; Comeche, B.; Chamorro, S.; Monge-Maillo, B.; Moreno-Guillén, S.; López-Vélez, R. COVID-19 ID Team Coronavirus disease 2019 and geographical area of origin. Clin. Microbiol. Infect. 2020, 27, 632.e1–632.e5. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística. Available online: https://www.ine.es/prensa/cp_j2020_p.pdf (accessed on 23 March 2021).
- Guijarro, C.; Pérez-Fernández, E.; González-Piñeiro, B.; Meléndez, V.; Goyanes, M.J.; Renilla, M.E.; Casas, M.L.; Sastre, I.; Velasco, M.; Investigadores COVID Alcorcón (Colaboradores); et al. Differential risk for COVID-19 in the first wave of the disease among migrants from several areas of the world living in Spain. Rev. Clin. Esp. 2020, 221, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Tirupathi, R.; Muradova, V.; Shekhar, R.; Salim, S.A.; Al-Tawfiq, J.A.; Palabindala, V. COVID-19 disparity among racial and ethnic minorities in the US: A cross sectional analysis. Travel Med. Infect. Dis. 2020, 38, 101904. [Google Scholar] [CrossRef] [PubMed]
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S.; et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths: A Systematic Review. Ann. Intern. Med. 2021, 174, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Ravenell, J.; Adhikari, S.; Butler, M.; Cook, T.; Francois, F.; Iturrate, E.; Jean-Louis, G.; Jones, S.A.; Onakomaiya, D.; et al. Assessment of Racial/Ethnic Disparities in Hospitalization and Mortality in Patients With COVID-19 in New York City. JAMA Netw. Open 2020, 3, e2026881. [Google Scholar] [CrossRef] [PubMed]
- Kabarriti, R.; Brodin, N.P.; Maron, M.I.; Guha, C.; Kalnicki, S.; Garg, M.K.; Racine, A.D. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Netw. Open 2020, 3, e2019795. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Solomon, N.; de Lemos, J.A.; Das, S.R.; Morrow, D.A.; Bradley, S.M.; Elkind, M.S.V.; Williams Iv, J.H.; Holmes, D.; Matsouaka, R.A.; et al. Racial and Ethnic Differences in Presentation and Outcomes for Patients Hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation 2020, 143, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Ho, A.; Shah, M.D.; Shetgiri, R. Trends in Mortality From COVID-19 and Other Leading Causes of Death Among Latino vs. White Individuals in Los Angeles County, 2011–2020. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.V.; Triant, V.A.; Bunda, B.A.; Selvaggi, C.A.; Shinnick, D.J.; He, W.; Lu, F.; Porneala, B.C.; Cao, T.; Lubitz, S.A.; et al. Massachusetts general hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. PLoS ONE 2020, 15, e0244270. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Kidwai-Khan, F.; Tate, J.P.; Park, L.S.; King, J.T.; Skanderson, M.; Hauser, R.G.; Schultze, A.; Jarvis, C.I.; Holodniy, M.; et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 2020, 17, e1003379. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.P.; Reid, N.J.; Som, A.; Li, M.D.; Hyle, E.P.; Dugdale, C.M.; Lang, M.; Betancourt, J.R.; Deng, F.; Mendoza, D.P.; et al. Racial and Ethnic Disparities in Disease Severity on Admission Chest Radiographs among Patients Admitted with Confirmed Coronavirus Disease 2019: A Retrospective Cohort Study. Radiology 2020, 297, E303–E312. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Menéndez, M.; Trigo, E.; Borobia, A.M.; Arsuaga, M.; de la Calle-Prieto, F.; de Miguel Buckley, R.; Lago, M.; Arribas, J.R.; COVID@HULP Working Group. Presenting characteristics and outcomes of migrants in a cohort of hospitalized patients with COVID-19: Does the origin matter? Travel Med. Infect. Dis. 2021, 42, 102027. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.R.; Ezzeldin, T.; Siddiqui, I.A.; Alzahrani, M.S.; Alghamdi, M.A.; Alotaibi, W.H.; Almutairi, M.Z.; Alqannas, N.K. Correlation of Racial Effect with Severity of Disease and In-Hospital Outcome in Individuals Diagnosed with COVID-19. Int. J. Clin. Pract. 2021, e14383. [Google Scholar] [CrossRef]

| European [n = 614] | Latin American [n = 82] | p | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), years | 67 (54–79) | 52 (40–64) | <0.001 |
| Age ≥ 65 years old, % (N) | 54.7% (336/614) | 24.4% (20/82) | <0.001 |
| Males, % (N) | 53.9% (331/614) | 48.8% (40/82) | 0.382 |
| Nosocomial, % (N) | 5.5% (34/614) | 1.2% (1/82) | 0.109 |
| Long-term care resident, % (N) | 4.9% (30/614) | 1.2% (1/82) | 0.161 |
| Health professional, % (N) | 5% (31/614) | 6.1% (5/82) | 0.601 |
| Comorbidities | |||
| Hypertension, % (N) | 52.9% (325/614) | 19.5% (16/82) | <0.001 |
| Diabetes, % (N) | 24.3% (149/614) | 9.8% (8/82) | 0.003 |
| Current or former smoker, % (N) | 20.6% (70/339) | 8.6% (5/58) | 0.087 |
| Obesity, % (N) | 39.6% (168/424) | 37.8% (17/45) | 0.810 |
| Chronic respiratory disease, % (N) | 20.4% (125/612) | 12.3% (10/81) | 0.084 |
| Immunosuppression, % (N) | 5.7% (35/614) | 2.5% (2/81) | 0.298 |
| Charlson comorbidity index, median (IQR) | 3 (1–5) | 1 (0–2) | <0.001 |
| Charlson index ≥ 3, % (N) | 56.67% (348/614) | 17% (14/82) | <0.001 |
| 10-year expected survival † | 53.2 (0.9–90) | 90.2 (0.95–95) | 0.016 |
| Clinical Presentation | |||
| Median time (IQR) from symptom to hospitalization, days ‡ | 6 (3–9) | 7 (4–9) | 0.180 |
| Fever, % (N) | 70.4% (430/611) | 70.7% (58/82) | 0.947 |
| Cough, % (N) | 66.4% (405/610) | 75.6% (62/82) | 0.094 |
| Dyspnea, % (N) | 52.9% (324/612) | 57.3% (47/82) | 0.456 |
| Diarrhea, % (N) | 27% (164/607) | 25.9% (21/81) | 0.835 |
| Confusion, % (N) | 8.8% (54/560) | 3.7% (3/82) | 0.080 |
| Fatigue, % (N) | 44.2% (265/599) | 37.5% (30/80) | 0.253 |
| Myalgias-arthralgias, % (N) | 26.4% (159/603) | 30%(24/80) | 0.491 |
| Anosmia-dysgeusia, % (N) | 17% (102/599) | 25% (20/80) | 0.081 |
| Initial Assessment | |||
| WHO ordinal scale §, % (N) | |||
| 4 5 6 7 8 | 70.0 (430) 8.6 (53) 1.5 (9) 5.0 (31) 14.9 (91) | 76.8 (63) 6.1 (5) 0.0 (0) 9.8 (8) 7.3 (6) | 0.015 |
| Oximetry < 94% at room air, % (N) | 45.8% (27/59) | 33.8% (27/80) | 0.151 |
| PaO2:FiO2, median (IQR) | 338 (281–404) | 375 (300–416) | 0.083 |
| Respiratory rate, breaths/min, median (IQR) | 16 (16–23) | 17 (16–20) | 0.813 |
| Systolic BP, mmHg, median (IQR) | 132 (116–146) | 129 (116–143) | 0.614 |
| Diastolic BP, mmHg, median (IQR) | 78 (67–89) | 81 (70–89) | 0.122 |
| Temperature, °C, median (IQR) | 36.9 (36.3–37.7) | 36.7 (36.4–37.5) | 0.339 |
| Heart rate, beats/min, median (IQR) | 92 (80–103) | 94 (81–105) | 0.221 |
| eGFR, mL/min/m2, median (IQR) | 78 (53–90) | 90 (72–90) | <0.001 |
| Lymphocytes, per mm3, median (IQR) | 1000 (730–1380) | 1200 (830–1560) | 0.011 |
| Lymphopenia, % (N) | 49.1% (298/607) | 34.6% (28/81) | 0.014 |
| C-reactive protein > 10 mg/dL, % (N) | 172/605 (28.4) | 25/82 (30.5) | 0.699 |
| Procalcitonin > 0.5 ng/mL, % (N) | 10.5% (58/550) | 8.0% (6/75) | 0.495 |
| Ferritin > 500 mg/L, % (N) | 58.2% (331/569) | 53.3% (40/75) | 0.425 |
| Lactate dehydrogenase > 250 U/L, % (N) | 62.3% (335/538) | 62.8% (49/78) | 0.925 |
| D-dimers > 1 mg/mL, % (N) | 33.6% (184/548) | 21.5% (17/79) | 0.03 |
| Interleukin 6 ≥ 10 pg/mL, % (N) | 75.5% (349/462) | 62.7% (42/67) | 0.025 |
| Troponin T > 14 ng/L, % (N) | 39.1% (211/540) | 9.3% (7/75) | <0.001 |
| Brain natriuretic peptide > 125 pg/mL, % (N) | 53.6% (288/537) | 26.0% (19/73) | <0.001 |
| Potassium mmol/L, median (IQR) | 4 (3.7–4.4) | 4 (3.8–4.3) | 0.790 |
| Opacities > 50% of lung surface on X-rays, % (N) | 23.5% (112/476) | 33.3% (21/63) | 0.090 |
| Treatment | |||
| Antibiotic use for >48 h, % (N) | 77.4% (261/337) | 75.9% (44/58) | 0.790 |
| Corticosteroids, % (N) | 61.9% (380/614) | 67.1% (55/82) | 0.362 |
| Remdesivir, % (N) | 9.5% (32/336) | 6.9% (4/58) | 0.521 |
| Tocilizumab, % (N) | 22.9% (139/606) | 21% (17/81) | 0.694 |
| European [n = 69 *] | Latin American [n = 69 *] | p | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), years | 59 (44–68) | 54 (43–66) | 0.178 |
| Males, % (N) | 62.3% (43/69) | 53.6% (37/69) | 0.301 |
| Nosocomial, % (N) | 1.4% (1/69) | 1.4% (1/69) | 1.000 |
| Long-term care resident, % (N) | 2.9% (2/69) | 1.4% (1/69) | 1.000 |
| Health professional, % (N) | 2.9% (2/69) | 5.8% (4/69) | 0.681 |
| Comorbidities | |||
| Hypertension, % (N) | 31.9% (22/69) | 18.8% (13/69) | 0.078 |
| Diabetes, % (N) | 15.9% (11/69) | 8.7% (6/69) | 0.195 |
| Current or former smoker, % (N) | 25.7% (9/35) | 9.8% (5/58) | 0.062 |
| Obesity, % (N) | 34% (17/50) | 34.3% (12/35) | 0.978 |
| Chronic respiratory disease, % (N) | 17.4% (12/69) | 14.7% (10/69) | 0.669 |
| Immunosuppression, % (N) | 8.7% (6/69) | 0.0% (0/69) | 0.028 |
| Charlson comorbidity index, median (IQR) | 2 (0–3) | 1 (0–2) | 0.260 |
| 10-year expected survival † | 90 (0.9–97) | 90 (0.9–96) | 0.517 |
| Clinical Presentation | |||
| Median time (IQR) from symptom to hospitalization, days ‡ | 7.0 (4.5–8.5) | 7.0 (4.5–9.5) | 0.867 |
| Fever, % (N) | 81.2% (56/69) | 72.5% (50/69) | 0.226 |
| Cough, % (N) | 84.1% (58/69) | 79.7% (55/69) | 0.507 |
| Dyspnea, % (N) | 63.2% (43/69) | 59.4% (41/69) | 0.647 |
| Diarrhea, % (N) | 37.7% (26/69) | 26.5% (18/68) | 0.160 |
| Confusion, % (N) | 5.8% (4/69) | 4.3% (3/69) | 0.698 |
| Fatigue, % (N) | 55.9% (38/68) | 38.2% (26/68) | 0.039 |
| Myalgias-arthralgias, % (N) | 36.8% (25/68) | 29.4% (20/68) | 0.362 |
| Anosmia-dysgeusia, % (N) | 29.4% (20/68) | 29.4% (20/68) | 1.000 |
| Initial Assessment | |||
| WHO ordinal scale § | |||
| 4, % (N) 5, % (N) 6, % (N) 7, % (N) 8, % (N) | 31.9% (22/69) 11.6% (8/69) 10.1% (7/69) 23.2% (16/69) 23.2% (16/69) | 79.7% (55/69) 4.3% (3/69) 0.0% (0/69) 7.2% (5/69) 8.7% (6/69) | <0.001 |
| WHO ordinal scale > 4 | 68.1% (47/69) | 20.3% (14/69) | <0.001 |
| Oximetry < 94% at room air, % (N) | 46.4% (32/69) | 33.3% (23/69) | 0.118 |
| PaO2:FiO2, median (IQR) | 338 (276–376) | 383 (300–420) | 0.030 |
| Respiratory rate, breaths/min, median (IQR) | 19 (16–25) | 16 (16–20) | 0.172 |
| Systolic BP, mmHg, median (IQR) | 128 (113–142) | 130 (116–145) | 0.474 |
| Diastolic BP, mmHg, median (IQR) | 80 (69–88) | 80 (70–89) | 0.564 |
| Temperature, °C, median (IQR) | 37.0 (36.4–37.8) | 36.7 (36.4–37.5) | 0.134 |
| Heart rate, beats/min, median (IQR) | 95 (87–103) | 94 (81–105) | 0.639 |
| eGFR, ml/min/m2, median (IQR) | 88 (70–90) | 90 (75–90) | 0.253 |
| Lymphopenia % (N) | 49.3% (34/69) | 37.7% (26/69) | 0.170 |
| C-reactive protein > 10 mg/dL % (N) | 34.8% (24/69) | 29.0% (20/69) | 0.465 |
| Procalcitonin > 0.5 ng/mL, % (N) | 9.0% (6/67) | 7.7% (5/65) | 0.793 |
| Ferritin > 500 mg/L, %, (N) | 79.1% (53/67) | 58.5% (38/65) | 0.010 |
| Lactate dehydrogenase > 250 U/L, % (N) | 76.5% (52/68) | 64.7% (44/68) | 0.132 |
| D-dimers > 1 mg/mL, % (N) | 27.5% (19/69) | 21.7% (15/69) | 0.429 |
| Interleukin 6 ≥ 10 pg/mL, % (N) | 79.2% (42/53) | 37.9% (36/58) | 0.048 |
| Troponin T > 14 ng/L, % (N) | 21.7% (15/69) | 8.7% (6/69) | 0.033 |
| Brain natriuretic peptide > 125 pg/mL, % (N) | 37.3% (25/67) | 22.7% (15/66) | 0.067 |
| Potassium mmol/L, median (IQR) | 4.2 (3.8–4.4) | 4.0 (3.8–4.3) | 0.212 |
| Opacities > 50% of lung surface on X-rays, % (N) | 40.6% (28/69) | 47.8% (33/69) | 0.391 |
| Treatment | |||
| Antibiotic use for >48 h | 32/35 (91.4%) | 39/51 (76.5%) | 0.073 |
| Corticosteroids | 49/69 (71.0%) | 47/69 (68.1%) | 0.711 |
| Remdesivir | 0/35 (9.5%) | 3/51 (5.9%) | 0.267 |
| Tocilizumab | 36/69 (52.2%) | 11/68 (16.2%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero-Rodriguez, S.; Moreno-Pérez, O.; Ramos, J.M.; García, M.; Boix, V.; Reus, S.; Torrus, D.; Chico-Sánchez, P.; Sánchez-Payá, J.; Aldana-Macias, F.; et al. Latin American Origin Is Not Associated with Worse Outcomes among Hospitalized Patients with COVID-19 in a Public Healthcare System. Microorganisms 2021, 9, 1772. https://doi.org/10.3390/microorganisms9081772
Otero-Rodriguez S, Moreno-Pérez O, Ramos JM, García M, Boix V, Reus S, Torrus D, Chico-Sánchez P, Sánchez-Payá J, Aldana-Macias F, et al. Latin American Origin Is Not Associated with Worse Outcomes among Hospitalized Patients with COVID-19 in a Public Healthcare System. Microorganisms. 2021; 9(8):1772. https://doi.org/10.3390/microorganisms9081772
Chicago/Turabian StyleOtero-Rodriguez, Silvia, Oscar Moreno-Pérez, Jose Manuel Ramos, Mar García, Vicente Boix, Sergio Reus, Diego Torrus, Pablo Chico-Sánchez, José Sánchez-Payá, Fernando Aldana-Macias, and et al. 2021. "Latin American Origin Is Not Associated with Worse Outcomes among Hospitalized Patients with COVID-19 in a Public Healthcare System" Microorganisms 9, no. 8: 1772. https://doi.org/10.3390/microorganisms9081772