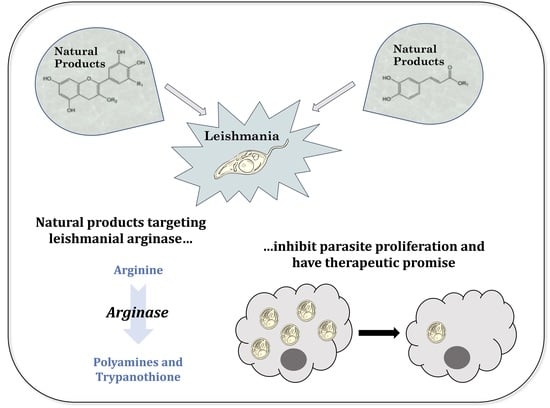
Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise
Abstract
:1. Introduction to Leishmania
2. Natural Products as an Antileishmanial Drugs
3. Arginase as a Promising Therapeutic Target
4. Natural Products That Target Arginase in Leishmania
4.1. Quercetin
4.1.1. Inhibition of Recombinant Arginase and Docking Studies
4.1.2. Other Cellular Targets of Quercetin
| Quercetin Target | Leishmania Species | Evidence | Reference |
|---|---|---|---|
| Arginase | L. amazonensis | - Inhibition of recombinant enzyme activity - In silico docking studies | [65,66,67] |
| Trypanothione synthetase-amidase and trypanothione reductase | L. tropica | - In silico docking studies | [68] |
| Ribonucleotide reductase | L. donovani | - Reduced enzyme activity in intracellular amastigotes | [69] |
| Topoisomerase II | L. donovani L. panamensis | - Inhibition of catalytic activity on minicircle DNA | [70,71] |
| Topoisomerase I | L. donovani | - Binding to recombinant enzyme - Inhibition of plasmid DNA relaxation | [72,73] |
| Cathepsin L-like rCPB2.8 | L. mexicana | - Inhibition of recombinant enzyme activity | [74] |
| gBP21 (RNA editing) | L. donovani | - In silico affinity for enzyme | [76] |
| Acetylcholinesterase | L. infantum chagasi | - Inhibition of enzyme activity | [75] |
4.1.3. In Vitro Activity against Promastigotes and Axenic Amastigotes
4.1.4. In Vitro Efficacy against Intracellular Amastigotes
4.1.5. Rodent Infectivity Studies
4.1.6. Pharmacokinetic Considerations for Quercetin
4.1.7. Summary of Quercetin Studies
4.2. Fisetin
4.3. Polyphenolic Compounds in Green Tea
4.3.1. Inhibition of Recombinant Arginase and Docking Studies
4.3.2. Effect on Morphology and Cellular Metabolism
4.3.3. In Vitro Activity against Promastigotes
4.3.4. In Vitro Efficacy against Intracellular Amastigotes
4.3.5. Murine Infectivity Studies
4.3.6. Pharmacokinetic Considerations for Green Tea Polyphenols
4.3.7. Summary of Green Tea Polyphenols
4.4. Resveratrol
4.5. Cinnamic Acid Derivatives
4.5.1. Inhibition of Recombinant Arginase and Docking Studies
4.5.2. In Vitro Activity against Promastigotes
| Compound | Parasite Species | EC50 | Reference |
|---|---|---|---|
| Caffeic acid | L. amazonensis | 5.2 μM after 72 h | [79] |
| L. amazonensis | >500 μM after 72 h | [19] | |
| L. infantum | 61 μM | [114] | |
| L. donovani | 59 μM | [20] | |
| Rosmarinic acid | L. amazonensis | 0.6 μM after 72 h | [79] |
| L. amazonensis | 61 μM after 72 h | [19] | |
| L. infantum | 57 μM | [114] | |
| L. donovani | 16 μM | [20] | |
| Chlorogenic acid | L. amazonensis | 0.5 μM after 72 h | [79] |
| L. amazonensis | >500 μM after 72 h | [19] | |
| L. donovani | 54 μM | [20] | |
| Isoverbascoside | L. amazonensis | 14 μM after 24 h | [19] |
| Verbascoside | L. amazonensis | 19 μM after 72 h | [19] |
| L. amazonensis | 19 μM | [132] | |
| n-butanolic fraction of S. cayennensis extract | L. amazonensis | 51 μg/mL after 72 h | [133] |
4.5.3. In Vitro Efficacy against Intracellular Amastigotes
4.5.4. Murine Infectivity Studies
4.5.5. Pharmacokinetic Considerations for Cinnamic Acid Derivatives
4.5.6. Summary of Cinnamic Acid Derivatives
4.6. Senna Spectabilis Extracts and Compounds
4.7. Extracts from Sambucus Ebulus and Urtica Dioica
5. Future Outlook for Arginase Inhibitors as Anti-Leishmanial Therapeutics
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | Catechin |
| EC | Epicatechin |
| ECG | Epicatechin gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-3-gallate |
| eIF5A | Eukaryotic translation initiation factor 5A |
| FL-Bu | n-butanol fraction |
| FL-DCM | Dichloromethane fraction |
| GA | Gallic acid |
| GCG | Gallocatechin gallate |
| IFN | Interferon |
| IL | Interleukin |
| Th1 | T-helper type 1 |
| TNF | Tumor necrosis factor |
References
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef]
- Desjeux, P. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 239–243. [Google Scholar] [CrossRef]
- Hotez, P.J. The rise of leishmaniasis in the twenty-first century. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 421–422. [Google Scholar] [CrossRef] [Green Version]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.M.; Werbovetz, K.A. Natural Products from Plants as Drug Candidates and Lead Compounds against Leishmaniasis and Trypanosomiasis. Curr. Med. Chem. 2006, 13, 2571–2598. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 10151, 951–970. [Google Scholar] [CrossRef]
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The Role of Natural Products in Drug Discovery and Development against Neglected Tropical Diseases. Molecules 2016, 22, 58. [Google Scholar] [CrossRef] [Green Version]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural Products as a Source for Treating Neglected Parasitic Diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A. Plant-derived compounds in treatment of leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites. Toxic. Assess. 2007, 393, 1–122. [Google Scholar] [CrossRef]
- Feher, M.; Schmidt, J.M. Property Distributions: Differences between Drugs, Natural Products, and Molecules from Combinatorial Chemistry. J. Chem. Inf. Comput. Sci. 2002, 43, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciências 2019, 91, e20190105. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saudagar, P. Flavones reversibly inhibit Leishmania donovani tyrosine aminotransferase by binding to the catalytic pocket: An integrated in silico-in vitro approach. Int. J. Biol. Macromol. 2020, 164, 2987–3004. [Google Scholar] [CrossRef]
- Mercado-Camargo, J.; Cervantes-Ceballos, L.; Vivas-Reyes, R.; Pedretti, A.; Serrano-García, M.L.; Gómez-Estrada, H. Homology Modeling of Leishmanolysin (gp63) from Leishmania panamensis and Molecular Docking of Flavonoids. ACS Omega 2020, 5, 14741–14749. [Google Scholar] [CrossRef]
- Khademvatan, S.; Eskandari, K.; Hazrati-Tappeh, K.; Rahim, F.; Foroutan, M.; Yousefi, E.; Asadi, N. In silico and in vitro comparative activity of green tea components against Leishmania infantum. J. Glob. Antimicrob. Resist. 2019, 18, 187–194. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Brogi, S.; Grillo, A.; Campiani, G.; Gemma, S.; Vieira, P.C.; Maquiaveli, C.D.C. Cinnamic acids derived compounds with antileishmanial activity target Leishmania amazonensis arginase. Chem. Biol. Drug Des. 2019, 93, 139–146. [Google Scholar] [CrossRef]
- Antwi, C.A.; Amisigo, C.M.; Adjimani, J.P.; Gwira, T.M. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLoS Negl. Trop. Dis. 2019, 13, e0007206. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and Bioefficacy of Polyphenols in Humans. Ii. Review of 93 Intervention Studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Abdelkawy, K.S.; Lack, K.; Elbarbry, F.A. Pharmacokinetics and Pharmacodynamics of Promising Arginase Inhibitors. Eur. J. Drug Metab. Pharmacokinet. 2016, 42, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.B.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef] [Green Version]
- Girard-Thernier, C.; Pham, T.-N.; Demougeot, C. The Promise of Plant-Derived Substances as Inhibitors of Arginase. Mini Rev. Med. Chem. 2015, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.I.; Laranjeira-Silva, M.F.; Muxel, S.M.; Floeter-Winter, L.M. The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr. Opin. Microbiol. 2019, 52, 110–115. [Google Scholar] [CrossRef]
- Balana-Fouce, R.; Calvo-Alvarez, E.; Alvarez-Velilla, R.; Prada, C.F.; Perez-Pertejo, Y.; Reguera, R.M. Role of Trypanosomatid’s Arginase in Polyamine Biosynthesis and Pathogenesis. Mol. Biochem. Parasitol. 2012, 181, 85–93. [Google Scholar] [CrossRef]
- Colotti, G.; Ilari, A. Polyamine metabolism in Leishmania: From arginine to trypanothione. Amino Acids 2011, 40, 269–285. [Google Scholar] [CrossRef]
- Da Silva, M.F.; Floeter-Winter, L.M. Arginase in Leishmania. Subcell Biochem. 2014, 74, 103–117. [Google Scholar]
- Ilari, A.; Fiorillo, A.; Baiocco, P.; Poser, E.; Angiulli, G.; Colotti, G. Targeting polyamine metabolism for finding new drugs against leishmaniasis: A review. Mini Rev. Med. Chem. 2015, 15, 243–252. [Google Scholar] [CrossRef]
- Muxel, S.M.; Aoki, J.I.; Fernandes, J.C.R.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Acuna, S.M.; Muller, K.E.; Vanderlinde, R.H.; Floeter-Winter, L.M. Arginine and Polyamines Fate in Leishmania Infection. Front Microbiol. 2017, 8, 2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessenda, G.; da Silva, J.S. Arginase and Its Mechanisms in Leishmania Persistence. Parasite Immunol. 2020, 42, e12722. [Google Scholar] [CrossRef] [PubMed]
- Boitz, J.M.; Gilroy, C.A.; Olenyik, T.D.; Paradis, D.; Perdeh, J.; Dearman, K.; Davis, M.J.; Yates, P.A.; Li, Y.; Riscoe, M.K.; et al. Arginase Is Essential for Survival of Leishmania donovani Promastigotes but Not Intracellular Amastigotes. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boitz, J.M.; Yates, P.A.; Kline, C.; Gaur, U.; Wilson, M.E.; Ullman, B.; Roberts, S.C. Leishmania donovani Ornithine Decarboxylase Is Indispensable for Parasite Survival in the Mammalian Host. Infect. Immun. 2008, 77, 756–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, M.F.L.; Zampieri, R.A.; Muxel, S.M.; Beverley, S.M.; Floeter-Winter, L.M. Leishmania amazonensis Arginase Compartmentalization in the Glycosome Is Important for Parasite Infectivity. PLoS ONE 2012, 7, e34022. [Google Scholar] [CrossRef] [Green Version]
- Gaur, U.; Roberts, S.C.; Dalvi, R.P.; Corraliza, I.; Ullman, B.; Wilson, M.E. An Effect of Parasite-Encoded Arginase on the Outcome of Murine Cutaneous Leishmaniasis. J. Immunol. 2007, 179, 8446–8453. [Google Scholar] [CrossRef]
- Gilroy, C.; Olenyik, T.; Roberts, S.C.; Ullman, B. Spermidine Synthase Is Required for Virulence of Leishmania donovani. Infect. Immun. 2011, 79, 2764–2769. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.A. Polyamines in protozoan pathogens. J. Biol. Chem. 2018, 293, 18746–18756. [Google Scholar] [CrossRef] [Green Version]
- Reguera, R.M.; Balaña-Fouce, R.; Showalter, M.; Hickerson, S.; Beverley, S.M. Leishmania major lacking arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol. Biochem. Parasitol. 2009, 165, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Ullman, B.; Ullman, S.R.A.B. Parasite Polyamines as Pharmaceutical Targets. Curr. Pharm. Des. 2017, 23, 3325–3341. [Google Scholar] [CrossRef]
- Roberts, S.C.; Tancer, M.J.; Polinsky, M.R.; Gibson, K.M.; Heby, O.; Ullman, B. Arginase Plays a Pivotal Role in Polyamine Precursor Metabolism in Leishmania. J. Biol. Chem. 2004, 279, 23668–23678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyfe, P.K.; Oza, S.L.; Fairlamb, A.H.; Hunter, W.N. Leishmania Trypanothione Synthetase-Amidase Structure Reveals a Basis for Regulation of Conflicting Synthetic and Hydrolytic Activities. J. Biol. Chem. 2008, 283, 17672–17680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairlamb, A.H. Trypanothione metabolism and rational approaches to drug design. Biochem. Soc. Trans. 1990, 18, 717–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairlamb, A.H.; Cerami, A. Metabolism and Functions of Trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol. Biochem. Parasitol. 2016, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Jhingran, A.; Singh, S.; Tyagi, N.; Park, M.H.; Srinivasan, N.; Roberts, S.C.; Madhubala, R. Identification and Characterization of a Novel Deoxyhypusine Synthase in Leishmania donovani. J. Biol. Chem. 2010, 285, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Chawla, B.; Kumar, R.R.; Tyagi, N.; Subramanian, G.; Srinivasan, N.; Park, M.H.; Madhubala, R. A Unique Modification of the Eukaryotic Initiation Factor 5A Shows the Presence of the Complete Hypusine Pathway in Leishmania donovani. PLoS ONE 2012, 7, e33138. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H. The Post-Translational Synthesis of a Polyamine-Derived Amino Acid, Hypusine, in the Eukaryotic Translation Initiation Factor 5A (eIF5A). J. Biochem. 2006, 139, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Lee, Y.B.; Joe, Y.A. Hypusine is essential for eukaryotic cell proliferation. Biol. Signals 1997, 6, 115–123. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.D.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.; Lahiri, A.; Lahiri, A.; Chakravortty, D. Modulation of the Arginase Pathway in the Context of Microbial Pathogenesis: A Metabolic Enzyme Moonlighting as an Immune Modulator. PLoS Pathog. 2010, 6, e1000899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniesta, V.; Carcelén, J.; Molano, I.; Peixoto, P.M.V.; Redondo, E.; Parra, P.; Mangas, M.; Monroy, I.; Campo, M.L.; Nieto, C.G.; et al. Arginase I Induction during Leishmania major Infection Mediates the Development of Disease. Infect. Immun. 2005, 73, 6085–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniesta, V.; Gomez-Nieto, L.C.; Corraliza, I. The Inhibition of Arginase by N(Omega)-Hydroxy-L-Arginine Controls the Growth of Leishmania inside Macrophages. J. Exp. Med. 2001, 193, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Gomez-Nieto, L.C.; Molano, I.; Mohedano, A.; Carcelen, J.; Miron, C.; Alonso, C.; Corraliza, I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002, 24, 113–118. [Google Scholar] [CrossRef]
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef]
- Kropf, P.; Herath, S.; Weber, V.; Modolell, M.; Muller, I. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 2003, 25, 439–447. [Google Scholar] [CrossRef]
- Badirzadeh, A.; Etaheri, T.; Taslimi, Y.; Abdossamadi, Z.; Heidari-Kharaji, M.; Gholami, E.; Sedaghat, B.; Niyyati, M.; Rafati, S. Arginase activity in pathogenic and non-pathogenic species of Leishmania parasites. PLoS Negl. Trop. Dis. 2017, 11, e0005774. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, H.; Sadeghipour, P.; Taslimi, Y.; Habibzadeh, S.; Zali, F.; Zahedifard, F.; Rahmati, J.; Kamyab, K.; Ghandi, N.; Zamanian, A.; et al. Comparing Acute and Chronic Human Cutaneous Leishmaniasis Caused by Leishmania Major and Leishmania Tropica Focusing on Arginase Activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2118–2121. [Google Scholar] [CrossRef]
- Müller, I.; Hailu, A.; Choi, B.-S.; Abebe, T.; Fuentes, J.M.; Munder, M.; Modolell, M.; Kropf, P. Age-Related Alteration of Arginase Activity Impacts on Severity of Leishmaniasis. PLoS Negl. Trop. Dis. 2008, 2, e235. [Google Scholar] [CrossRef] [Green Version]
- Nahidi, S.; Gholami, E.; Taslimi, Y.; Habibzadeh, S.; Seyed, N.; Daverpanah, E.; Ghanadan, A.; Rafati, S.; Etaheri, T. The outcome of arginase activity inhibition in BALB/c mice hosting Leishmania tropica. Parasite Immunol. 2019, 42, e12691. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, E.L.; Ullman, B.; Roberts, S.C.; Dixit, U.G.; Wilson, M.E.; Hai, Y.; Christianson, D.W. Crystal structure of arginase from Leishmania mexicana and implications for the inhibition of polyamine biosynthesis in parasitic infections. Arch. Biochem. Biophys. 2013, 535, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, Y.; Christianson, D.W. Crystal Structures of Leishmania Mexicana Arginase Complexed with Alpha, Alpha-Disubstituted Boronic Amino-Acid Inhibitors. Acta Crystallogr. Sect. Struct. Biol. Commun. 2016, 72, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.R.; Maquiaveli Cdo, C.; Magalhaes, P.P. The Leishmanicidal Flavonols Quercetin and Quercitrin Target Leishmania (Leishmania) Amazonensis Arginase. Exp. Parasitol. 2012, 130, 183–188. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Brogi, S.; Lucon-Júnior, J.F.; Campiani, G.; Gemma, S.; Maquiaveli, C.D.C. Dietary polyphenols rutin, taxifolin and quercetin related compounds target Leishmania amazonensis arginase. Food Funct. 2019, 10, 3172–3180. [Google Scholar] [CrossRef]
- Manjolin, L.C.; dos Reis, M.B.; Maquiaveli Cdo, C.; Santos-Filho, O.A.; da Silva, E.R. Dietary Flavonoids Fisetin, Luteolin and Their Derived Compounds Inhibit Arginase, a Central Enzyme in Leishmania (Leishmania) Amazonensis Infection. Food Chem. 2013, 141, 2253–2262. [Google Scholar] [CrossRef] [Green Version]
- Mehwish, S.; Khan, H.; Rehman, A.U.; Khan, A.U.; Khan, M.A.; Hayat, O.; Ahmad, M.; Wadood, A.; -Ullah, N. Natural compounds from plants controlling leishmanial growth via DNA damage and inhibiting trypanothione reductase and trypanothione synthetase: An in vitro and in silico approach. 3 Biotech 2019, 9, 303. [Google Scholar] [CrossRef]
- Sen, G.; Mukhopadhyay, S.; Ray, M.; Biswas, T. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. J. Antimicrob. Chemother. 2008, 61, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Mittra, B.; Saha, A.; Chowdhury, A.R.; Pal, C.; Mandal, S.; Mukhopadhyay, S.; Bandyopadhyay, S.; Majumder, H.K. Luteolin, an Abundant Dietary Component Is a Potent Anti-leishmanial Agent that Acts by Inducing Topoisomerase II-mediated Kinetoplast DNA Cleavage Leading to Apoptosis. Mol. Med. 2000, 6, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Cortázar, T.M.; Coombs, G.H.; Walker, S. Leishmania panamensis: Comparative inhibition of nuclear DNA topoisomerase II enzymes from promastigotes and human macrophages reveals anti-parasite selectivity of fluoroquinolones, flavonoids and pentamidine. Exp. Parasitol. 2007, 116, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Sen, N.; Roy, A.; Dasgupta, S.B.; Ganguly, A.; Mohanta, B.C.; Dinda, B.; Majumder, H.K. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: Activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res. 2006, 34, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean-Moreno, V.; Rojas, R.; Goyeneche, D.; Coombs, G.H.; Walker, J. Leishmania donovani: Differential activities of classical topoisomerase inhibitors and antileishmanials against parasite and host cells at the level of DNA topoisomerase I and in cytotoxicity assays. Exp. Parasitol. 2006, 112, 21–30. [Google Scholar] [CrossRef]
- De Sousa, L.R.; Wu, H.; Nebo, L.; Fernandes, J.B.; Silva, M.F.D.G.D.; Kiefer, W.; Schirmeister, T.; Vieira, P.C. Natural products as inhibitors of recombinant cathepsin L of Leishmania mexicana. Exp. Parasitol. 2015, 156, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vila-Nova, N.S.; De Morais, S.M.; Falcão, M.J.; Bevilaqua, C.M.; Rondon, F.C.; Wilson, M.E.; Vieira, Í.G.; Andrade, H.F. Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds of Dimorphandra gardneriana and Platymiscium floribundum, native plants from Caatinga biome. Pesqui. Veterinária Bras. 2012, 32, 1164–1168. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, G.C.; Yousuf Ansari, M.; Dikhit, M.R.; Kannan, M.; Rana, S.; Das, P. Structure Prediction of Gbp21 Protein of L. Donovani and Its Molecular Interaction. J. Biomol. Struct. Dyn. 2014, 32, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Del Rayo Camacho, M.; Phillipson, J.D.; Croft, S.L.; Marley, D.; Kirby, G.C.; Warhurst, D.C. Assessment of the Antiprotozoal Activity of Galphimia Glauca and the Isolation of New nor-Secofriedelanes and nor-Friedelanes. J. Nat. Prod. 2002, 65, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive Oxygen Species Production and Mitochondrial Dysfunction Contribute to Quercetin Induced Death in Leishmania amazonensis. PLoS ONE 2011, 6, e14666. [Google Scholar] [CrossRef]
- Montrieux, E.; Perera, W.H.; García, M.; Maes, L.; Cos, P.; Monzote, L. In vitro and in vivo activity of major constituents from Pluchea carolinensis against Leishmania amazonensis. Parasitol. Res. 2014, 113, 2925–2932. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Assolini, J.P.; Panis, C.; Kian, D.; Yamauchi, L.M.; Simão, A.N.C.; Casagrande, R.; Pinge-Filho, P.; et al. Quercetin promotes antipromastigote effect by increasing the ROS production and anti-amastigote by upregulating Nrf2/HO-1 expression, affecting iron availability. Biomed. Pharmacother. 2019, 113, 108745. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca-Silva, F.; Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive Oxygen Species Production by Quercetin Causes the Death of Leishmania amazonensis Intracellular Amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oyeleye, S.I.; Dada, F.A.; Olasehinde, T.A.; Oboh, G. Modulatory effect of quercetin and its glycosylated form on key enzymes and antioxidant status in rats penile tissue of paroxetine-induced erectile dysfunction. Biomed. Pharmacother. 2018, 107, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Bordage, S.; Pham, T.-N.; Zedet, A.; Gugglielmetti, A.-S.; Nappey, M.; Demougeot, C.; Girard, C. Investigation of Mammal Arginase Inhibitory Properties of Natural Ubiquitous Polyphenols by Using an Optimized Colorimetric Microplate Assay. Planta Med. 2016, 83, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Muzitano, M.; Falcão, C.; Cruz, E.; Bergonzi, M.C.; Bilia, A.R.; Vincieri, F.; Rossi-Bergmann, B.; Costa, S.S. Oral Metabolism and Efficacy of Kalanchoe pinnata Flavonoids in a Murine Model of Cutaneous Leishmaniasis. Planta Med. 2008, 75, 307–311. [Google Scholar] [CrossRef]
- Sousa-Batista, A.J.; Poletto, F.; Philipon, C.I.M.S.; Guterres, S.S.; Pohlmann, A.R.; Rossi-Bergmann, B. Lipid-core nanocapsules increase the oral efficacy of quercetin in cutaneous leishmaniasis. Parasitology 2017, 144, 1769–1774. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Linkevičiūtė, A.; Misiūnas, A.; Naujalis, E.; Barauskas, J. Preparation and characterization of quercetin-loaded lipid liquid crystalline systems. Colloids Surf. B Biointerfaces 2015, 128, 296–303. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; López-Ahumada, G.A.; Carvajal-Millan, E.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Preparation and Characterization of Quercetin-Loaded Zein Nanoparticles by Electrospraying and Study of In Vitro Bioavailability. J. Food Sci. 2019, 84, 2883–2897. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; Detakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Elbarbry, F.A.; Ung, A.; Abdelkawy, K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharmacogn. Mag. 2018, 13, S895–S899. [Google Scholar] [PubMed]
- Choi, J.-S.; Li, X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int. J. Pharm. 2005, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans1. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef]
- Pal, H.C.; Pearlman, R.L.; Afaq, F. Fisetin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 213–244. [Google Scholar] [CrossRef]
- Adinehbeigi, K.; Jalali, M.R.; Shahriari, A.; Bahrami, S. In vitro antileishmanial activity of fisetin flavonoid via inhibition of glutathione biosynthesis and arginase activity in Leishmania infantum. Pathog. Glob. Health 2017, 111, 176–185. [Google Scholar] [CrossRef]
- Touil, Y.S.; Auzeil, N.; Boulinguez, F.; Saighi, H.; Regazzetti, A.; Scherman, D.; Chabot, G.G. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem. Pharmacol. 2011, 82, 1731–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touil, Y.; Seguin, J.; Scherman, D.; Chabot, G.G. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother. Pharmacol. 2010, 68, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shia, C.-S.; Tsai, S.-Y.; Kuo, S.-C.; Hou, Y.-C.; Chao, P.-D.L. Metabolism and Pharmacokinetics of 3,3′,4′,7-Tetrahydroxyflavone (Fisetin), 5-Hydroxyflavone, and 7-Hydroxyflavone and Antihemolysis Effects of Fisetin and Its Serum Metabolites. J. Agric. Food Chem. 2009, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.; Sistla, R. Enhanced Oral Bioavailability and Anticancer Efficacy of Fisetin by Encapsulating as Inclusion Complex with Hpbetacd in Polymeric Nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Dos Reis, M.B.; Manjolin, L.C.; Maquiaveli Cdo, C.; Santos-Filho, O.A.; Da Silva, E.R. Inhibition of Leishmania (Leishmania) Amazonensis and Rat Arginases by Green Tea Egcg, (+)-Catechin and (-)-Epicatechin: A Comparative Structural Analysis of Enzyme-Inhibitor Interactions. PLoS ONE 2013, 8, e78387. [Google Scholar] [CrossRef]
- Inacio, J.D.F.; Gervazoni, L.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. The Effect of (-)-Epigallocatechin 3-O—Gallate In Vitro and In Vivo in Leishmania braziliensis: Involvement of Reactive Oxygen Species as a Mechanism of Action. PLoS Negl. Trop. Dis. 2014, 8, e3093. [Google Scholar] [CrossRef] [Green Version]
- Inacio, J.D.; Canto-Cavalheiro, M.M.; Menna-Barreto, R.F.S.; Almeida-Amaral, E.E. Mitochondrial damage contribute to epigallocatechin-3-gallate induced death in Leishmania amazonensis. Exp. Parasitol. 2012, 132, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Das, S.; Roy, S.; Ghosh, A.K.; Sardar, A.H.; Verma, S.; Saini, S.; Singh, R.; Abhishek, K.; Kumar, A.; et al. Deprivation of L-Arginine Induces Oxidative Stress Mediated Apoptosis in Leishmania donovani Promastigotes: Contribution of the Polyamine Pathway. PLoS Negl. Trop. Dis. 2016, 10, e0004373. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.G.; Nascimento, A.M.; Henriques, B.O.; Chávez-Fumagalli, M.A.; Franca, J.R.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.; Lage, D.P.; Rodrigues, L.B.; et al. Antileishmanial activity of standardized fractions of Stryphnodendron obovatum (Barbatimão) extract and constituent compounds. J. Ethnopharmacol. 2015, 165, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Saduqi, M.; Sharifi, I.; Babaei, Z.; Keyhani, A.; Mostafavi, M.; Parizi, M.H.; Ghasemian, M.; Bamorovat, M.; Sharifi, F.; Aflatoonian, M.R.; et al. Anti-Leishmanial and Immunomodulatory Effects of Epigallocat-echin 3-O-Gallate on Leishmania tropica: Apoptosis and Gene Expression Profiling. Iran. J. Parasitol. 2019, 14, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.D.M.; Brito, L.M.; Souza, A.C.; Queiroz, B.C.S.H.; De Carvalho, T.P.; Batista, J.F.; Oliveira, J.S.D.S.M.; De Mendonça, I.L.; Lira, S.R.D.S.; Chaves, M.H.; et al. Gallic and ellagic acids: Two natural immunomodulator compounds solve infection of macrophages by Leishmania major. Naunyn Schmiedeberg’s Arch. Pharmacol. 2017, 390, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.R.; Oliveira, D.M.P.; Amaral, A.C.F.; Jesus, J.B.; Sodero, A.C.R.; Souza, A.M.T.; Supuran, C.; Vermelho, A.B.; Rodrigues, I.A.; Pinheiro, A.S. Leishmania infantum arginase: Biochemical characterization and inhibition by naturally occurring phenolic substances. J. Enzym. Inhib. Med. Chem. 2019, 34, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. In Vitro and in Vivo Effects of (−)-Epigallocatechin 3-O-gallate on Leishmania amazonensis. J. Nat. Prod. 2013, 76, 1993–1996. [Google Scholar] [CrossRef]
- Sosa, A.M.; Álvarez, A.M.; Bracamonte, E.; Korenaga, M.; Marco, J.D.; Barroso, P.A. Efficacy of Topical Treatment with (−)-Epigallocatechin Gallate, A Green Tea Catechin, in Mice with Cutaneous Leishmaniasis. Molecules 2020, 25, 1741. [Google Scholar] [CrossRef]
- Kolodziej, H.; Radtke, O.A.; Kiderlen, A.F. Stimulus (polyphenol, IFN-γ, LPS)-dependent nitric oxide production and antileishmanial effects in RAW 264.7 macrophages. Phytochemistry 2008, 69, 3103–3110. [Google Scholar] [CrossRef]
- Radtke, O.A.; Kiderlen, A.F.; Kayser, O.; Kolodziej, H. Gene Expression Profiles of Inducible Nitric Oxide Synthase and Cytokines in Leishmania major -Infected Macrophage-Like RAW 264.7 Cells Treated with Gallic Acid. Planta Med. 2004, 70, 924–928. [Google Scholar] [CrossRef]
- Alves, M.M.D.M.; Arcanjo, D.D.R.; Figueiredo, K.A.; Oliveira, J.S.D.S.M.; Viana, F.J.C.; Coelho, E.D.S.; Lopes, G.L.N.; Gonçalves, J.C.R.; Carvalho, A.L.M.; Rizzo, M.D.S.; et al. Gallic and Ellagic Acids Are Promising Adjuvants to Conventional Amphotericin B for the Treatment of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of Dosing Condition on the Oral Bioavailability of Green Tea Catechins after Single-Dose Administration of Polyphenon E in Healthy Individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of Green Tea Polyphenols and the Biological Activities of Those Metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.D.; Dos Santos, J.A.; Machado, P.A.; Alves, L.A.; Laque, L.C.; De Souza, V.C.; Coimbra, E.S.; Capriles, P.V.S.Z. Insights about resveratrol analogs against trypanothione reductase of Leishmania braziliensis: Molecular modeling, computational docking and in vitro antileishmanial studies. J. Biomol. Struct. Dyn. 2018, 37, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Pallerla, D.S.R.; Kaur, P.K.; Babu, N.K.; Singh, S. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb. Pathog. 2014, 66, 14–23. [Google Scholar] [CrossRef]
- Ferreira, C.; Soares, D.C.; Nascimento, M.T.C.D.; Pinto-Da-Silva, L.; Sarzedas, C.G.; Tinoco, L.; Saraiva, E.M. Resveratrol Is Active against Leishmania amazonensis:In VitroEffect of Its Association with Amphotericin B. Antimicrob. Agents Chemother. 2014, 58, 6197–6208. [Google Scholar] [CrossRef] [Green Version]
- Kedzierski, L.; Curtis, J.M.; Kaminska, M.; Jodynis-Liebert, J.; Murias, M. In vitro antileishmanial activity of resveratrol and its hydroxylated analogues against Leishmania major promastigotes and amastigotes. Parasitol. Res. 2007, 102, 91–97. [Google Scholar] [CrossRef]
- Lucas, I.; Kolodziej, H. In Vitro Antileishmanial Activity of Resveratrol Originates from its Cytotoxic Potential against Host Cells. Planta Medica 2012, 79, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.I.; Koo, B.H.; Hong, D.; Kwon, H.J.; Hoe, K.L.; Won, M.H.; Kim, Y.M.; Lim, H.K.; Ryoo, S. Resveratrol is an arginase inhibitor contributing to vascular smooth muscle cell vasoconstriction via increasing cytosolic calcium. Mol. Med. Rep. 2019, 19, 3767–3774. [Google Scholar] [CrossRef]
- Guzman, J. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B. Cinnamic Acid Derivatives and their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Maquiaveli, C.C.; Lucon-Júnior, J.F.; Brogi, S.; Campiani, G.; Gemma, S.; Vieira, P.C.; Silva, E.R. Verbascoside Inhibits Promastigote Growth and Arginase Activity of Leishmania amazonensis. J. Nat. Prod. 2016, 79, 1459–1463. [Google Scholar] [CrossRef]
- Maquiaveli, C.D.C.; e Sá, A.M.O.; Vieira, P.C.; da Silva, E.R. Stachytarpheta Cayennensis Extract Inhibits Promastigote and Amastigote Growth in Leishmania Amazonensis Via Parasite Arginase Inhibition. J. Ethnopharmacol. 2016, 192, 108–113. [Google Scholar] [CrossRef]
- Cury, T.A.C.; Yoneda, J.S.; Zuliani, J.P.; Soares, A.M.; Stábeli, R.G.; Calderon, L.A.; Ciancaglini, P. Cinnamic acid derived compounds loaded into liposomes: Antileishmanial activity, production standardisation and characterisation. J. Microencapsul. 2015, 32, 467–477. [Google Scholar] [CrossRef]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Gračan, R.; Nemrava, J. Oxidative stress, polarization of macrophages and tumour angiogenesis: Efficacy of caffeic acid. Chem. Interact. 2016, 256, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Maquiaveli, C.D.C.; Rochetti, A.L.; Fukumasu, H.; Vieira, P.C.; da Silva, E.R. Antileishmanial Activity of Verbascoside: Selective Arginase Inhibition of Intracellular Amastigotes of Leishmania (Leishmania) Amazonensis with Resistance Induced by Lps Plus Ifn-Gamma. Biochem. Pharmacol. 2017, 127, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.P.; Tomaz, D.C.; De Souza, L.A.; Onofre, T.S.; De Menezes, W.A.; Almeida-Silva, J.; Suarez-Fontes, A.M.; De Almeida, M.R.; Da Silva, A.M.; Bressan, G.C.; et al. Synthesis of cinnamic acid derivatives and leishmanicidal activity against Leishmania braziliensis. Eur. J. Med. Chem. 2019, 183, 111688. [Google Scholar] [CrossRef]
- Vale-Costa, S.; Gouveia, J.C.; Pérez, B.; Silva, T.; Teixeira, C.; Gomes, P.; Gomes, S. N-Cinnamoylated Aminoquinolines as Promising Antileishmanial Agents. Antimicrob. Agents Chemother. 2013, 57, 5112–5115. [Google Scholar] [CrossRef] [Green Version]
- Masic, A.; Hernandez, A.M.V.; Hazra, S.; Glaser, J.; Holzgrabe, U.; Hazra, B.; Schurigt, U. Cinnamic Acid Bornyl Ester Derivatives from Valeriana wallichii Exhibit Antileishmanial In Vivo Activity in Leishmania major-Infected BALB/c Mice. PLoS ONE 2015, 10, e0142386. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Clemente, L.B.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.-T. Absorption of Hydroxycinnamates in Humans after High-Bran Cereal Consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S.; Shimizu, M. Transepithelial Transport of P-Coumaric Acid and Gallic Acid in Caco-2 Cell Monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2317–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Moghadasian, M.H. Bioavailability of hydroxycinnamates: A brief review of in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of Microbial Metabolites of Dietary Polyphenols in Rats: Is the Brain Their Target Destination? ACS Chem. Neurosci. 2015, 6, 1341–1352. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Yasui, H.; Sakurai, H. Suppressive Effect of Caffeic Acid and Its Derivatives on the Generation of Uva-Induced Reactive Oxygen Species in the Skin of Hairless Mice and Pharmacokinetic Analysis on Organ Distribution of Caffeic Acid in Ddy Mice. Photochem. Photobiol. 2006, 82, 668–676. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [Green Version]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Melo, G.M.D.A.; Silva, M.C.R.; Guimarães, T.P.; Pinheiro, K.M.; Da Matta, C.B.B.; Queiroz, A.C.; Pivatto, M.; Bolzani, V.S.; Alexandre-Moreira, M.S.; Viegas, C.J. Leishmanicidal activity of the crude extract, fractions and major piperidine alkaloids from the flowers of Senna spectabilis. Phytomedicine 2014, 21, 277–281. [Google Scholar] [CrossRef]
- Lacerda, R.B.M.; Freitas, T.R.; Martins, M.M.; Teixeira, T.L.; Da Silva, C.V.; Candido, P.A.; De Oliveira, R.J.; Júnior, C.V.; Bolzani, V.D.S.; Danuello, A.; et al. Isolation, leishmanicidal evaluation and molecular docking simulations of piperidine alkaloids from Senna spectabilis. Bioorganic Med. Chem. 2018, 26, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Daneshfard, B.; Emtiazy, M.; Khiveh, A.; Hashempur, M.H. Biological Effects and Clinical Applications of Dwarf Elder (Sambucus ebulus L.): A Review. J. Evid. Based Integr. Med. 2017, 22, 996–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadkhodamasoum, S.; Bineshian, F.; Karimipour, A.; Tavakoli, P.; Foroutan, M.; Ghaffarifar, F.; Molaei, S. Comparison the effects of Sambucus ebulus leaf and fruit extracts on Leishmania major in-vitro. Infect. Disord. Drug Targets 2019, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Kharaji, M.; Fallah-Omrani, V.; Badirzadeh, A.; Mohammadi-Ghalehbin, B.; Nilforoushzadeh, M.A.; Masoori, L.; Montakhab-Yeganeh, H.; Zare, M. Sambucus ebulus extract stimulates cellular responses in cutaneous leishmaniasis. Parasite Immunol. 2018, 41, e12605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Badirzadeh, A.; Heidari-Kharaji, M.; Fallah-Omrani, V.; Dabiri, H.; Araghi, A.; Chirani, A.S. Antileishmanial activity of Urtica dioica extract against zoonotic cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0007843. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.-N.; Bordage, S.; Pudlo, M.; Demougeot, C.; Thai, K.-M.; Girard, C. Cinnamide Derivatives as Mammalian Arginase Inhibitors: Synthesis, Biological Evaluation and Molecular Docking. Int. J. Mol. Sci. 2016, 17, 1656. [Google Scholar] [CrossRef]





| Leishmania Species | Type of Leishmaniasis | Geographical Distribution | Old vs. New World |
|---|---|---|---|
| L. major | Cutaneous | West Africa to Central Asia | Old world |
| L. tropica | Eastern Mediterranean, Middle East, North India, Afghanistan, and northeast and South Africa | ||
| L. donovani | Visceral | South Asia, East Africa | |
| L. infantum/ L. infantum chagasi | Middle East, Afghanistan, Iran, Pakistan | ||
| L. braziliensis | Cutaneous, Mucosal | South America | New world |
| L. amazonensis | South America | ||
| L. mexicana | Cutaneous | South America | |
| L. infantum/ L. infantum chagasi | Visceral | Mexico and Central and Latin America |
| Leishmania Species | EC50 Promastigotes or Efficacy | EC50 Axenic Amastigotes | Reference |
|---|---|---|---|
| L. amazonensis | 31 μM (48 h) | [78] | |
| 0.7 μM (72 h) | [79] | ||
| L. donovani | 3.3 μM | [81] | |
| 46 μM (24 h) | [70] | ||
| 64 μM | [77] | ||
| 16% growth inhibition in 512 μM quercetin | [73] | ||
| L. infantum chagasi | 86 μM (24 h) | [75] | |
| L. tropica | 603 μM (72 h) | 454 μM (72 h) | [68] |
| L. braziliensis | ≤50% viability in 48 μM quercetin at 24 h | [80] |
| Leishmania Species | Macrophages Derived from | EC50 or Efficacy | Reference |
|---|---|---|---|
| L. amazonensis | Peritoneal Swiss mice | 3.4 μM | [82] |
| Peritoneal female BALB/c mice | 4.3 μM | [79] | |
| L. braziliensis | Peritoneal female BALB/c mice | Statistically significant reduction of infectivity at 48 μM and 70 μM concentrations | [80] |
| L. donovani | Peritoneal BALB/c mice | 70% reduction of intracellular amastigotes at 46 μM | [70] |
| L. infantum chagasi | RAW 264.7 | 35 μM | [75] |
| Leishmania Species | Rodent | Route of Administration and Efficacy | Reference |
|---|---|---|---|
| L. donovani | Golden hamsters | - Quercetin delivered (orally) free or in combination with serum albumin (injected); quercetin doses: 5 to 50 mg/kg - Free quercetin: 75% reduction in splenic parasite load - Quercetin + serum albumin: 95% reduction in splenic parasite load | [69] |
| BALB/c mice | - Quercetin dose (intraperitoneally): 30 mg/kg (5 doses) - 15% inhibition of liver parasite load | [81] | |
| L. amazonensis | BALB/c mice | - Quercetin and lipid-core nanocapsules containing quercetin (orally); 16 mg/kg (51 doses) - Free quercetin: Lesion size reduced by 38%, parasite loads reduced by 71% - Quercetin nanocapsules: Lesion size reduced by 64%, parasite loads reduced by 91% | [86] |
| BALB/c mice | - Quercetin dose (intragastric gavage): 16 mg/kg (30 doses) - reduced parasite burden by 76% | [85] | |
| BALB/c mice | - Quercetin dose (intralesion injections): 30 mg/kg (5 doses) - reduced lesion size after 5 and 6 weeks | [79] |
| Leishmania Secies | EC50 | Reference | |||||
|---|---|---|---|---|---|---|---|
| EGCG | ECG | GCG | C | EC | GA | ||
| L. infantum | 28 μM | 75 μM | 94 μM | 212 μM | [17] | ||
| 395 μM | [114] | ||||||
| L. donovani | 42 μM | 20 μM | [81] | ||||
| L. amazonensis | 63 μM | [109] | |||||
| 37 μM | 145 μM | 10 μM | [111] | ||||
| L. braziliensis | 278 μM | [108] | |||||
| L. tropica | 190 μM | [112] | |||||
| L. major | 97 μM | [113] | |||||
| Leishmania Species | EC50 | Macrophages Derived from | Reference | ||||
|---|---|---|---|---|---|---|---|
| EGCG | GCG | GC | C | GA | |||
| L. amazonensis | 130 μM | 148 μM | 213 μM | THP1 | [116] | ||
| 1.6 μM | Peritoneal Swiss mice | [115] | |||||
| L. braziliensis | 3.4 μM | Peritoneal Swiss mice | [108] | ||||
| L. tropica | 46 μM | J774 | [112] | ||||
| L. major | 30 μM | Peritoneal BALB/c mice | [113] | ||||
| L. infantum | 287 μM | RAW 264.7 | [114] | ||||
| Compound | Parasite Species | IC50 Ki % Inhibition | Type of Inhibition | Reference |
|---|---|---|---|---|
| Caffeic acid | L. amazonensis | IC50 1.5 μM Ki 0.5 μM | Competitive | [19] |
| L. infantum | 57% inhibition at 100 μM concentration | [114] | ||
| Rosmarinic acid | L. amazonensis | IC50 2.1 μM Ki 1.8 μM | Competitive | [19] |
| L. infantum | 71% inhibition at 100 μM concentration | [114] | ||
| Chlorogenic acid | L. amazonensis | IC50 8.3 μM Ki 5 μM | Competitive | [19] |
| Cryptochlorogenic acid | L. amazonensis | IC50 11 μM Ki 12.3 μM | Noncompetitive | [19] |
| Isoverbascoside | L. amazonensis | IC50 2.3 μM Ki 1 μM | Noncompetitive | [19] |
| Verbascoside | L. amazonensis | Ki 0.7 μM | Competitive | [19] |
| L. amazonensis | Ki 0.7 μM | Competitive | [132] | |
| N-butanolic fraction of S. cayennensis extract | L. amazonensis | IC50 1.2 μg/mL | [133] |
| Compound | Parasites Species | EC50 | Macrophage | Reference |
|---|---|---|---|---|
| Caffeic acid | L. infantum | 22 μM | RAW 264.7 | [114] |
| L. amazonensis | 16 μM | Peritoneal, from female BALB/c mice | [79] | |
| Rosmarinic acid | L. infantum | 7.9 μM | RAW 264.7 | [114] |
| L. amazonensis | 4.8 μM | Peritoneal, from female BALB/c mice | [79] | |
| Chlorogenic acid | L. amazonensis | 5.3 μM | Peritoneal, from female BALB/c mice | [79] |
| Verbascoside | L. amazonensis | 32 μM | RAW 264.7 | [136] |
| n-butanolic fraction of S. cayennensis extract | L. amazonensis | 32 μg/mL | RAW 264.7 | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267. https://doi.org/10.3390/microorganisms9020267
Carter NS, Stamper BD, Elbarbry F, Nguyen V, Lopez S, Kawasaki Y, Poormohamadian R, Roberts SC. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms. 2021; 9(2):267. https://doi.org/10.3390/microorganisms9020267
Chicago/Turabian StyleCarter, Nicola S., Brendan D. Stamper, Fawzy Elbarbry, Vince Nguyen, Samuel Lopez, Yumena Kawasaki, Reyhaneh Poormohamadian, and Sigrid C. Roberts. 2021. "Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise" Microorganisms 9, no. 2: 267. https://doi.org/10.3390/microorganisms9020267