An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Phage Isolation
2.3. Adsorption Rate, Latent Period, and Phage Burst Size
2.4. Complex Hp Phage, Lactoferrin, and Hydroxyapatite Nanoparticles (Hp φ +LF-HA)
2.5. Measurement of Cell Viability
2.5.1. MTT Assay
2.5.2. Trypan Blue Test
2.5.3. NO2 Measurements
2.6. SEM Image
2.7. Helicobacter Pylori Culture, Gastric Cell Infection
2.8. Quantitative Gene Expression Analysis
2.9. ROS Detection Assay
3. Results
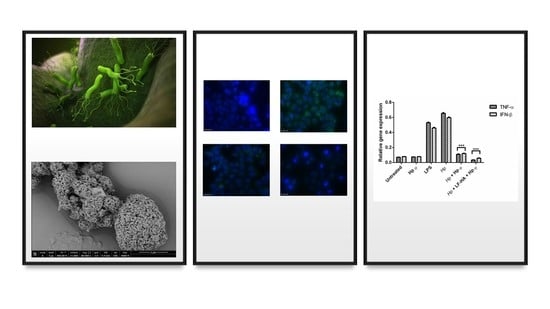
3.1. Phage Isolation
3.2. Hp φ +LF-HA Complex
3.3. Cytotoxic Activities of the Phage Hp φ
3.4. In Vitro Infection of AGS Cells with Helicobacter pylori and Treatment with Hp φ +LF-HA
3.5. ROS Detection Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, S.; Carra, G.; Sahin, U.; Hoy, B.; Rieder, G.; Wessler, S. Complex cellular responses of Helicobacter pylori-colonized gastric adenocarcinoma cells. Infect. Immun. 2011, 79, 2362–2371. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, A.; Marcos-Pinto, R.; Nairn, A.V.; dela Rosa, M.; Ferreira, R.M.; Junqueira-Neto, S.; Freitas, D.; Gomes, J.; Oliveira, P.; Santos, M.R.; et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1928–1939. [Google Scholar] [CrossRef] [Green Version]
- Camilo, V.; Sugiyama, T.; Touati, E. Pathogenesis of Helicobacter pylori infection. Helicobacter 2017, 22, e12405. [Google Scholar] [CrossRef]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Contaldi, F.; Capuano, F.; Fulgione, A.; Aiese Cigliano, R.; Sanseverino, W.; Iannelli, D.; Medaglia, C.; Capparelli, R. The hypothesis that Helicobacter pylori predisposes to Alzheimer’s disease is biologically plausible. Sci. Rep. 2017, 7, 7817. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. World J. Gastroenterol. 2018, 24, 3204. [Google Scholar]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Capparelli, R.; Nocerino, N.; Lanzetta, R.; Silipo, A.; Amoresano, A.; Giangrande, C.; Becker, K.; Blaiotta, G.; Evidente, A.; Cimmino, A.; et al. Bacteriophage-Resistant Staphylococcus aureus Mutant Confers Broad Immunity against Staphylococcal Infection in Mice. PLoS ONE 2010, 5, e11720. [Google Scholar] [CrossRef] [Green Version]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef]
- Górski, A.; Dąbrowska, K.; Miȩdzybrodzki, R.; Weber-Dąbrowska, B.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Borysowski, J. Phages and immunomodulation. Future Microbiol. 2017, 12, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Morozova, V.V.; Vlassov, V.V.; Tikunova, N.V. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front. Microbiol. 2018, 9, 1696. [Google Scholar] [CrossRef] [Green Version]
- Nobrega, F.L.; Costa, A.R.; Santos, J.F.; Siliakus, M.F.; Van Lent, J.W.M.; Kengen, S.W.M.; Azeredo, J.; Kluskens, L.D. Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Vinner, G.K.; Rezaie-Yazdi, Z.; Leppanen, M.; Stapley, A.G.F.; Leaper, M.C.; Malik, D.J. Microencapsulation of Salmonella-specific bacteriophage felix o1 using spray-drying in a ph-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals 2019, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, med.21572. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Ghiasi, B.; Sefidbakht, Y.; Rezaei, M. Hydroxyapatite for biomedicine and drug delivery. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 104, pp. 85–120. [Google Scholar]
- Li, T.T.; Ling, L.; Lin, M.C.; Jiang, Q.; Lin, Q.; Lin, J.H.; Lou, C.W. Properties and mechanism of hydroxyapatite coating prepared by electrodeposition on a braid for biodegradable bone scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Fulgione, A.; Ianniello, F.; Papaianni, M.; Contaldi, F.; Sgamma, T.; Giannini, C.; Pastore, S.; Velotta, R.; Della Ventura, B.; Roveri, N.; et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for Salmonella bacteriophages. Int. J. Nanomed. 2019, 14, 2219–2232. [Google Scholar] [CrossRef] [Green Version]
- Papaianni, M.; Cuomo, P.; Fulgione, A.; Albanese, D.; Gallo, M.; Paris, D.; Motta, A.; Iannelli, D.; Capparelli, R. Bacteriophages Promote Metabolic Changes in Bacteria Biofilm. Microorganisms 2020, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin Adsorbed onto Biomimetic Hydroxyapatite Nanocrystals Controlling—In Vivo—The Helicobacter pylori Infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [Green Version]
- Okuda, M.; Nakazawa, T.; Yamauchi, K.; Miyashiro, E.; Koizumi, R.; Booka, M.; Teraguchi, S.; Tamura, Y.; Yoshikawa, N.; Adachi, Y.; et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: A randomized, double-blind, placebo-controlled study. J. Infect. Chemother. 2005, 11, 265–269. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; Delagarza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [Green Version]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Tomasini, M.L.; Zanussi, S.; Sozzi, M.; Tedeschi, R.; Basaglia, G.; De Paoli, P. Heterogeneity of cag genotypes in Helicobacter pylori isolates from human biopsy specimens. J. Clin. Microbiol. 2003, 41, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Papaianni, M.; Contaldi, F.; Fulgione, A.; Woo, S.L.; Casillo, A.; Corsaro, M.M.; Parrilli, E.; Marcolungo, L.; Rossato, M.; Delledonne, M.; et al. Role of phage ϕ1 in two strains of Salmonella Rissen, sensitive and resistant to phage ϕ1. BMC Microbiol. 2018, 18, 208. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental Phage Therapy against Staphylococcus aureus in Mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Papaianni, M.; Paris, D.; Woo, S.L.; Fulgione, A.; Rigano, M.M.; Parrilli, E.; Tutino, M.L.; Marra, R.; Manganiello, G.; Casillo, A.; et al. Plant dynamic metabolic response to bacteriophage treatment after Xanthomonas campestris pv. campestris infection. Front. Microbiol. 2020, 11, 732. [Google Scholar] [CrossRef]
- Carrieri, R.; Manco, R.; Sapio, D.; Iannaccone, M.; Fulgione, A.; Papaianni, M.; de Falco, B.; Grauso, L.; Tarantino, P.; Ianniello, F.; et al. Structural data and immunomodulatory properties of a water-soluble heteroglycan extracted from the mycelium of an Italian isolate of Ganoderma lucidum. Nat. Prod. Res. 2017, 31, 2119–2125. [Google Scholar] [CrossRef]
- Papaianni, M.; Ricciardelli, A.; Fulgione, A.; d’Errico, G.; Zoina, A.; Lorito, M.; Woo, S.L.; Vinale, F.; Capparelli, R. Antibiofilm Activity of a Trichoderma Metabolite against Xanthomonas campestris pv. campestris, Alone and in Association with a Phage. Microorganisms 2020, 8, 620. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palomba, L.; Silvestri, C.; Imperatore, R.; Morello, G.; Piscitelli, F.; Martella, A.; Cristino, L.; Di Marzo, V. Negative regulation of leptin-induced reactive oxygen species (ROS) formation by cannabinoid CB1 receptor activation in hypothalamic neurons. J. Biol. Chem. 2015, 290, 13669–13677. [Google Scholar] [CrossRef] [Green Version]
- Papastergiou, V.; Georgopoulos, S.D.; Karatapanis, S. Treatment of Helicobacter pylori infection: Meeting the challenge of antimicrobial resistance. World J. Gastroenterol. 2014, 20, 9898–9911. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102. [Google Scholar] [CrossRef]
- Pellicano, R.; Ribaldone, D.G.; Caviglia, G.P. Strategies for Helicobacter pylori eradication in the year 2020. Saudi J. Gastroenterol. 2020, 26, 63–65. [Google Scholar]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. BioMetals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- Iafisco, M.; Di Foggia, M.; Bonora, S.; Prat, M.; Roveri, N. Adsorption and spectroscopic characterization of lactoferrin on hydroxyapatite nanocrystals. Dalt. Trans. 2011, 40, 820–827. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-Induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Górski, A.; Jonczyk-Matysiak, E.; Lusiak-Szelachowska, M.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Borysowski, J. The potential of phage therapy in sepsis. Front. Immunol. 2017, 8, 1783. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R., Jr.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [Green Version]
- Yokota, S.I.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect. Immun. 2010, 78, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative Stress Resulting From Helicobacter pylori Infection Contributes to Gastric Carcinogenesis. CMGH 2017, 3, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.Z.; Minohara, Y.; Xue, J.F.; Wang, J.; Reyes, V.E.; Patel, J.; Dirden-Kramer, B.; Boldogh, I.; Ernst, P.B.; Crowe, S.E. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect. Immun. 2007, 75, 4030–4039. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Kruzel, M.; Hwang, S.A.; Ba, X.; Boldogh, I. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef] [Green Version]







| Hp Samples | Hp φ |
|---|---|
| Patient 1 | - |
| Patient 2 | + |
| Patient 3 | + |
| Patient 4 | + |
| Patient 5 | + |
| Patient 6 | + |
| Patient 7 | + |
| Patient 8 | + |
| Patient 9 | + |
| Patient 10 | + |
| Patient 11 | + |
| Patient 12 | + |
| Patient 13 | + |
| Patient 14 | + |
| Patient 15 | - |
| Patient 16 | - |
| Patient 17 | + |
| Patient 18 | + |
| Patient 19 | + |
| Patient 20 | - |
| Patient 21 | - |
| Patient 22 | + |
| Patient 23 | + |
| Patient 24 | + |
| Patient 25 | + |
| Patient 26 | + |
| Patient 27 | + |
| Patient 28 | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuomo, P.; Papaianni, M.; Fulgione, A.; Guerra, F.; Capparelli, R.; Medaglia, C. An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization. Microorganisms 2020, 8, 1214. https://doi.org/10.3390/microorganisms8081214
Cuomo P, Papaianni M, Fulgione A, Guerra F, Capparelli R, Medaglia C. An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization. Microorganisms. 2020; 8(8):1214. https://doi.org/10.3390/microorganisms8081214
Chicago/Turabian StyleCuomo, Paola, Marina Papaianni, Andrea Fulgione, Fabrizia Guerra, Rosanna Capparelli, and Chiara Medaglia. 2020. "An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization" Microorganisms 8, no. 8: 1214. https://doi.org/10.3390/microorganisms8081214