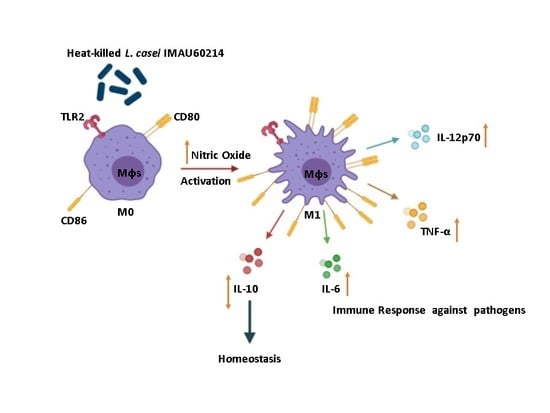
Evaluation of Immunomodulatory Activities of the Heat-Killed Probiotic Strain Lactobacillus casei IMAU60214 on Macrophages In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Preparation of Heat-Killed Probiotic Bacteria
2.2. Preparation and Treatment of Human Monocyte-Derived Macrophages (MDMs)
2.3. NO Production Assay
2.4. Quantification of Cytokine Release
2.5. Flow Cytometric Assay for Surface Marker Expression
2.6. Phagocytosis Assay
2.7. Assays with a Toll-Like Receptor 2 (TLR2)-Neutralizing Antibody
2.8. Statistical Analysis
3. Results
3.1. Purity and Morphological Identification of MDM Cells
3.2. Induction of NO Release from MDMs by Heat-Killed L. casei IMAU60214
3.3. Induction of Cytokine Release from MDMs by Heat-Killed L. casei IMAU60214
3.4. Induction of CD80 and CD86 Surface Expression by MDMs In Response to Heat-Killed L. casei IMAU60214
3.5. Induction of Macrophage Phagocytic Activity by Heat-Killed L. casei IMAU60214
3.6. Effects of L. casei IMAU60214 on Macrophages Are Dependent on Activation of Toll-Like Receptor 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Douillard, F.P.; de Vos, W.M. Functional genomics of lactic acid bacteria: From food to health. Microb. Cell Factories 2014, 13, S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Harris, H.M.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei Group: History and Health Related Applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aureli, P.; Carpuso, L.; Castellazzi, A.M.; Clerici, M.; Giovannini, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G.V. Probiotics and health; An evidence-based review. Pharmacol. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Stavropoulou, E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe 2011, 17, 369–374. [Google Scholar] [CrossRef]
- Elmadfa, I.; Klein, P.; Meyer, A.L. Immune-stimulating effects of lactic acid bacteria in vivo and in vitro. Proc. Nutr. 2010, 69, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.L.; Elmadfa, I.; Herbacek, I.; Micksche, M. Probiotic, as well as conventional yogurt, can enhance the stimulated production of proinflammatory cytokines. J. Hum. Nutr. Diet. 2007, 20, 590–598. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Pravin, M.; Muthusamy, V.S.; Bharathi, R.R.; Lakshmi, B.S. In vitro Investigation of the Immunomodulatory Potencial of Probiotic Lactobacillus casei. Probiotics Antimicrob Proteins 2013, 5, 51–58. [Google Scholar] [CrossRef]
- Liévin-Le, M.V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [Green Version]
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol. 2012, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Bay, B.H.; Lee, Y.K.; Lu, J.; Mahendran, R. Live and lyophilized Lactobacillus species elicit differential immunomodulatory effects on immune cells. FEMS Microbiol. Lett. 2010, 30, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, I.J.; Segers, M.E.; Verhoeven, T.L.; Dusselier, M.; Sels, B.F.; De Keersmaecker, S.C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic acid is an important microbe-associated molecular pattern of Lactobacillus rhamnosus GG. Microb. Cell Factories 2012, 15, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbs, G.; Newman, M.A. The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe-associated molecular patterns (MAMPs), in plant innate immunity. Mol. Plant Pathol. 2012, 13, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Losacco, A.; Carratelli, C.R. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol. Lett. 2013, 156, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.K.; He, B.; Wang, T.; Tran, D.Q.; Rhoads, J.M.; Liu, Y. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G231–G240. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.H.; Park, J.H.; Choi, S.Y.; Jeon, H.Y.; Park, J.I.; Kim, J.Y.; Ham, S.H.; Choi, Y.K. The Probiotic Lactobacillus Prevents Citrobacter rodentium-Induced Murine Colitis in a TLR2-Dependent Manner. J. Microbiol. Biotechnol. 2016, 26, 1333–1340. [Google Scholar] [CrossRef]
- Muzio, M.; Bosisio, D.; Polentarutti, N.; D’amico, G.; Stoppacciaro, A.; Mancinelli, R.; van’t Veer, C.; Penton-Rol, G.; Ruco, L.P.; Allavena, P.; et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: Selective expression of TLR3 in dendritic cells. J. Immunol. 2000, 164, 5998–6004. [Google Scholar]
- Jensen, H.; Drømtorp, S.M.; Axelsson, L.; Grimmer, S. Immunomodulation of monocytes by probiotic and selected lactic Acid bacteria. Probiotics Antimicrob. Proteins 2015, 7, 14–23. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Chomarat, P.; Banchereau, J.; Davoust, J.; Palucka, A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000, 1, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125, S53–S72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2015, 82, 1173–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.T.; Ngo, V.L.; Cho, Y.H.; Ko, E.J.; Hong, S.M.; Kim, K.H.; Jang, J.H.; Oh, J.S.; Park, M.K.; Kim, C.H.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar]
- Yasuda, E.; Serata, M.; Sako, T. Suppressive effect on activation of macrophages by Lactobacillus casei strain Shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl. Environ Microbiol. 2008, 74, 4746–4755. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.C.; Tomita, S.; Kleerebezem, M.; Bron, P.A. The quest for probiotic effector molecules-unraveling strain specificity at the molecular level. Pharmacol. Res. 2013, 69, 61–74. [Google Scholar] [CrossRef]
- Habil, N.; Al-Murrani, W.; Beal, J.; Foey, A.D. Probiotic bacterial strain differentially modulate macrophage cytokine production in a strain dependent and cell subset-specific manner. Benef. Microbes 2011, 2, 4283–4293. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno-Guerrero, S.S.; Ramírez-Pacheco, A.; García-Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [Green Version]
- Carpuso, L. Thirty Years of lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Dos Santos, T.; Alves Melo, T.; Almeida, M.E.; Passos Rezende, R.; Romano, C.C. Immunomodulatory Effects of Lactobacillus plantarum Lp62 on Intestinal Epithelial and Mononuclear Cells. Biomed Res. Int. 2016, 2016, 8404156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Nogales, A.; Algieri, F.; Vezza, T.; Garrido-Mesa, N.; Olivares, M.; Comalada, M.; Riccardi, C.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Galvez, J. The viability of Lactobacillus fermentum CECT5716 is not essential to exert intestinal anti-inflammatory properties. Food Funct. 2015, 6, 1176–1184. [Google Scholar] [CrossRef]
- Sashihara, T.; Sueki, N.; Ikegami, S. An analysis of the effectiveness of heat-killed lactic acid bacteria in alleviating allergic diseases. J. Dairy Sci. 2006, 89, 2846–2855. [Google Scholar] [CrossRef]
- Zhang, L.; Nan, L.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-a -induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 16178. [Google Scholar] [CrossRef]
- Jones-Carson, J.; Zweifel, A.E.; Tapscott, T.; Austin, C.; Brown, J.M.; Jones, K.L.; Voskuil, M.I.; Vázquez-Torres, A. Nitric oxide from IFNγ-primed macrophages modulates the antimicrobial activity of β-lactams against the intracellular pathogens Burkholderia pseudomallei and Non typhoidal Salmonella. PLoS Negl. Trop. Dis. 2014, 14, e3079. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses (Table of Contents). Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billack, B. Macrophage activation: Role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Pietilä, T.E.; Kekkonen, R.A.; Kankainen, M.; Latvala, S.; Pirhonen, J.; Österlund, P.; Korpela, R.; Julkunen, I. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes. 2012, 3, 510–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 577, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Fenton, T.M.; Czajkowska, B.I.; Klementowicz, J.E.; Travis, M.A. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012, 217, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Shin, J.S.; Lee, S.G.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR2-mediated activation. Int. Immunopharmacol. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, M.S.; Ji, G.E. Probiotic modulation of dendritic cells co-cultured with intestinal epithelial cells. World J. Gastroenterol. 2012, 18, 1308–1318. [Google Scholar] [CrossRef]
- Shida, K.; Nanno, M.; Nagata, S. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria: A possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes 2011, 2, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef]
- Sun, K.Y.; Xu, D.H.; Xie, C.; Plummer, S.; Tang, J.; Yang, X.F.; Ji, X.H. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 2017, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, N.; Okinaga, T.; Nishihara, T.; Maeda, T. Enhanced phagocytosis of Aggregatibacter actinomycetemcomitans cells by macrophages activated by a probiotic Lactobacillus strain. J. Dairy Sci. 2018, 101, 5789–5798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagihara, S.; Goto, H.; Hirota, T.; Fukuda, S.; Ohno, H.; Yamamoto, N. Lactobacillus acidophilus L-92 Cells Activate Expression of Immunomodulatory Genes in THP-1 Cells. Biosci. Microbiota Food Health 2014, 33, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Lang, F.; Zhao, N.; Guo, Y.; Zhang, H. Vaginal Lactobacilli Induce Differentiation of Monocytic Precursors Toward Langerhans-like Cells: In Vitro Evidence. Front. Immunol. 2018, 9, 2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Lee, T.S. Enhancement in ex vivo phagocytic capacity of peritoneal leukocytes in mice by oral delivery of various lactic-acid-producing bacteria. Curr. Microbiol. 2005, 50, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Prasad, J.; Gopal, P.K. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br. J. Nutr. 2000, 83, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, G.E.; Lambrecht, R.S. Augmentation of macrophage phagocytic activity by cell-free extracts of selected lactic acid-producing bacteria. J. Dairy Sci. 1993, 76, 2485–2492. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Du, S.; Liu, H.; Zhang, Y.; Wang, C.; Rong, F.; Jin, N. Evaluation of immunomodulatory activity of two potential probiotic Lactobacillus strains by in vivo tests. Anaerobe 2015, 35, 22–27. [Google Scholar] [CrossRef]
- Li, A.L.; Sun, Y.Q.; Du, P.; Meng, X.C.; Guo, L.; Li, S.; Zhang, C. The Effect of Lactobacillus actobacillus Peptidoglycan on Bovine β-Lactoglobulin-Sensitized Mice via TLR2/NF-κB Pathway. Iran. J. Allergy Asthma Immunol. 2017, 16, 147–158. [Google Scholar]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Kaji, R.; Kiyoshima-Shibata, J.; Tsujibe, S.; Nanno, M.; Shida, K. Short communication: Probiotic induction of interleukin-10 and interleukin-12 production by macrophages is modulated by co-stimulation with microbial components. J. Dairy Sci. 2018, 10, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 34561. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Ramírez, L.M.; Hernández-Ochoa, B.; Gómez-Manzo, S.; Marcial-Quino, J.; Cárdenas-Rodríguez, N.; Centeno-Leija, S.; García-Garibay, M. Evaluation of Immunomodulatory Activities of the Heat-Killed Probiotic Strain Lactobacillus casei IMAU60214 on Macrophages In Vitro. Microorganisms 2020, 8, 79. https://doi.org/10.3390/microorganisms8010079
Rocha-Ramírez LM, Hernández-Ochoa B, Gómez-Manzo S, Marcial-Quino J, Cárdenas-Rodríguez N, Centeno-Leija S, García-Garibay M. Evaluation of Immunomodulatory Activities of the Heat-Killed Probiotic Strain Lactobacillus casei IMAU60214 on Macrophages In Vitro. Microorganisms. 2020; 8(1):79. https://doi.org/10.3390/microorganisms8010079
Chicago/Turabian StyleRocha-Ramírez, Luz María, Beatriz Hernández-Ochoa, Saúl Gómez-Manzo, Jaime Marcial-Quino, Noemí Cárdenas-Rodríguez, Sara Centeno-Leija, and Mariano García-Garibay. 2020. "Evaluation of Immunomodulatory Activities of the Heat-Killed Probiotic Strain Lactobacillus casei IMAU60214 on Macrophages In Vitro" Microorganisms 8, no. 1: 79. https://doi.org/10.3390/microorganisms8010079