Viability and Virulence of Listeria monocytogenes in Poultry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Isolation and Identification of Listeria spp.
2.3. Quantification and Viability of Listeria monocytogenes Determined by q-PCR
2.4. Comparison between OCLA&PCR and v-PCR Techniques
2.5. Virulence Genes
2.6. Ability to Form Biofilm
2.7. Antibiotic Resistance
2.8. Statistical Analysis
3. Results
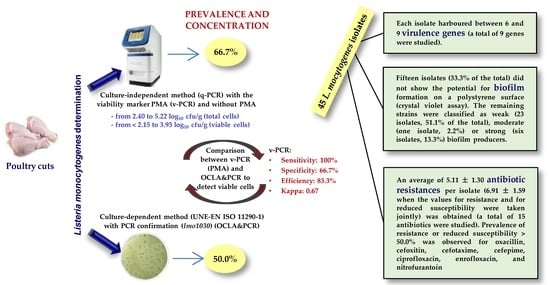
3.1. Prevalence of Listeria spp. and Listeria monocytogenes
3.2. Concentration of Viable Cells of Listeria monocytogenes
3.3. Comparison between OCLA&PCR and v-PCR
3.4. Virulence Genes
3.5. Potential for Biofilm Formation
3.6. Antibiotic Resistance
3.7. Relationships between Virulence Factors
4. Discussion
4.1. Prevalence of Listeria spp. and Listeria monocytogenes
4.2. Concentration of Viable Cells of Listeria monocytogenes
4.3. Comparison between OCLA&PCR and v-PCR
4.4. Virulence Genes
4.5. Potential for Biofilm Formation
4.6. Antibiotic Resistance
4.7. Relationships between Virulence Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwaiwu, O. An overview of Listeria species in Nigeria. Int. Food Res. J. 2015, 22, 455–464. [Google Scholar]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; García-Fernández, C.; Carballo, J.; Capita, R. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for twelve antimicrobials (biocides and antibiotics) in eight strains of Listeria monocytogenes. Biology 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, R.S.; Martin, K.G.; Keys, A.L.; Haney, C.J.; Shen, Y.; Smiley, R.D. 16S rRNA partial gene sequencing for the differentiation and molecular subtyping of Listeria species. Food Microbiol. 2013, 36, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ainsworth, A.J.; Austin, F.W.; Lawrence, M.L. Identification of Listeria innocua by PCR targeting a putative transcriptional regulator gene. FEMS Microbiol. Lett. 2003, 223, 205–210. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Ryser, E.T.; Marth, E.H. Listeria, Listeriosis, and Food Safety, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Le Monnier, A.; Brisse, S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.H.; Yeom, Y.S.; Shrivastav, A.; Lee, S.H.; Kim, Y.R.; Kim, H.Y. Simultaneous detection of Listeria species isolated from meat processed foods using multiplex PCR. Food Control 2013, 32, 659–664. [Google Scholar] [CrossRef]
- Barocci, S.; Calza, L.; Blasi, G.; Briscolini, S.; De Curtis, M.; Palombo, B.; Cucco, L.; Postacchini, M.; Sabbatini, M.; Graziosi, T.; et al. Evaluation of a rapid molecular method for detection of Listeria monocytogenes directly from enrichment broth media. Food Control 2008, 19, 750–756. [Google Scholar] [CrossRef]
- Ferrentino, G.; Tamburini, S.; Bath, K.; Foladori, P.; Spilimbergo, S.; Jousson, O. Application of culture-independent methods for monitoring Listeria monocytogenes inactivation on food products. Process Biochem. 2015, 50, 188–193. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, quantification and antibiotic resistance of Listeria monocytogenes in poultry preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- Josephson, K.L.; Gerba, C.P.; Pepper, I.L. Polymerase chain reaction detection of nonviable bacterial pathogens. Appl. Environ. Microbiol. 1993, 59, 3513–3515. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. Quantification using real-time PCR technology: Applications and limitations. Trends Mol. Med. 2002, 8, 257–260. [Google Scholar] [CrossRef]
- Nkuipou-Kenfack, E.; Engel, H.; Fakih, S.; Nocker, A. Improving efficiency of viability-PCR for selective detection of live cells. J. Microbiol. Methods 2013, 93, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Soejima, T.; Iida, K.I.; Qin, T.; Taniai, H.; Seki, M.; Takade, A.; Yoshida, S.I. Photoactivated ethidium monoazide directly cleaves bacterial DNA and is applied to PCR for discrimination of live and dead bacteria. Microbiol. Immunol. 2007, 51, 763–775. [Google Scholar] [CrossRef]
- Nogva, H.K.; Drømtorp, S.M.; Nissen, H.; Rudi, K. Ethidium monoazide for DNA-based differentiation of viable and dead bacteria by 5′-nuclease PCR. BioTechniques 2003, 34, 804–813. [Google Scholar] [CrossRef]
- Rey, M.D.L.Á.; Cap, M.; Favre, L.C.; Rodríguez Racca, A.; Dus Santos, M.J.; Vaudagna, S.R.; Mozgovoj, M. Evaluation of PMA-qPCR methodology to detect and quantify viable Shiga toxin-producing Escherichia coli in beef burgers. J. Food Process. Preserv. 2021, 45, e15338. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Johnson, D.I. Bacterial Pathogens and Their Virulence Factors, Bacterial Pathogens and Their Virulence Factors; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kubori, T.; Nagai, H. The Type IVB secretion system: An enigmatic chimera. Curr. Opin. Microbiol. 2016, 29, 22–29. [Google Scholar] [CrossRef]
- Colautti, A.; Arnoldi, M.; Comi, G.; Iacumin, L. Antibiotic resistance and virulence factors in lactobacilli: Something to carefully consider. Food Microbiol. 2022, 103, 103934. [Google Scholar] [CrossRef] [PubMed]
- Selvan, P.; Narendra Babu, R.; Sureshkumar, S.; Venkataramanujam, V. Microbial quality of retail meat products available in Chennai city. Am. J. Food Technol. 2007, 2, 55–59. [Google Scholar] [CrossRef]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative genomics of Listeria species. Science 2001, 294, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C.; Moreno, B.; García-Fernández, M.C. Occurrence of Listeria species in retail poultry meat and comparison of a cultural/immunoassay for their detection. Int. J. Food Microbiol. 2001, 65, 75–82. [Google Scholar] [CrossRef]
- Arslan, S.; Baytur, S. Prevalence and antimicrobial resistance of Listeria species and subtyping and virulence factors of Listeria monocytogenes from retail meat. J. Food Saf. 2019, 39, e12578. [Google Scholar] [CrossRef]
- Díez-García, M.; Capita, R.; Alonso-Calleja, C. Influence of serotype on the growth kinetics and the ability to form biofilms of Salmonella isolates from poultry. Food Microbiol. 2012, 31, 173–180. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standars Institute: Wayne, PA, USA, 2018. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org (accessed on 17 February 2023).
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI supplement VET08; Clinical and Laboratory Standars Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawewnce Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Hernández Lalinde, J.D.; Espinosa Castro, F.; Rodríguez, J.E.; Chacón Rangel, J.G.; Toloza Sierra, C.A.; Arenas Torrado, M.K.; Carrillo Sierra, S.M.; Bermúdez Pirela, V.J. Sobre el uso adecuado del coeficiente de correlación de Pearson: Definición, propiedades y suposiciones. Arch. Venez. Farmacol. Ter. 2018, 37, 587–595. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 17 February 2023).
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Gonçalves-Tenório, A.; Nunes Silva, B.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of pathogens in poultry meat: A meta-analysis of European published surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Amajoud, N.; Leclercq, A.; Soriano, J.M.; Bracq-Dieye, H.; El Maadoudi, M.; Senhaji, N.S.; Kounnoun, A.; Moura, A.; Lecuit, M.; Abrini, J. Prevalence of Listeria spp. and characterization of Listeria monocytogenes isolated from food products in Tetouan, Morocco. Food Control 2018, 84, 436–441. [Google Scholar] [CrossRef]
- Tao, T.; Chen, Q.; Bie, X.; Lu, F.; Lu, Z. Investigation on prevalence of Listeria spp. and Listeria monocytogenes in animal-derived foods by multiplex PCR assay targeting novel genes. Food Control 2017, 73, 704–711. [Google Scholar] [CrossRef]
- Zeinali, T.; Jamshidi, A.; Bassami, M.; Rad, M. Isolation and identification of Listeria spp. in chicken carcasses marketed in northeast of Iran. Int. Food Res. J. 2017, 24, 881–887. [Google Scholar]
- Soultos, N.; Koidis, P.; Madden, R.H. Presence of Listeria and Salmonella spp. in retail chicken in Northern Ireland. Lett. Appl. Microbiol. 2003, 37, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Vitas, A.I.; Aguado, V.; Garcia-Jalon, I. Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain). Int. J. Food Microbiol. 2004, 90, 349–356. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Esteves, A.; Panera-Martínez, S.; Capita, R.; Alonso-Calleja, C. Quantification of total and viable cells and determination of serogroups and antibiotic resistance patterns of Listeria monocytogenes in chicken meat from the North-Western Iberian peninsula. Antibiotics 2022, 11, 1828. [Google Scholar] [CrossRef]
- Aziz, S.A.A.A.; Mohamed, M.B.E.D. Prevalence, virulence genes, and antimicrobial resistance profile of Listeria monocytogenes isolated from retail poultry shops in Beni-Suef city, Egypt. J. Adv. Vet. Anim. Res. 2002, 7, 710–717. [Google Scholar] [CrossRef]
- Bilir Ormanci, F.S.; Erol, I.; Ayaz, N.D.; Iseri, O.; Sariguzel, D. Immunomagnetic separation and PCR detection of Listeria monocytogenes in turkey meat and antibiotic resistance of the isolates. Br. Poult. Sci. 2008, 49, 560–565. [Google Scholar] [CrossRef]
- Fallah, A.A.; Saei-Dehkordi, S.S.; Rahnama, M.; Tahmasby, H.; Mahzounieh, M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control 2012, 28, 327–332. [Google Scholar] [CrossRef]
- Kanarat, S.; Jitnupong, W.; Sukhapesna, J. Prevalence of Listeria monocytogenes in chicken production chain in Thailand. Thai J. Vet. Med. 2011, 41, 155–161. [Google Scholar] [CrossRef]
- Kuan, C.H.; Goh, S.G.; Loo, Y.Y.; Chang, W.S.; Lye, Y.L.; Tang, J.Y.H.; Nakaguchi, Y.; Nishibuchi, M.; Mahyudin, N.A.; Radu, S. Prevalence and quantification of Listeria monocytogenes in chicken offal at the retail level in Malaysia. Poult. Sci. 2013, 92, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Alaboudi, A.R.; Nesiar, E.A. Prevalence of Listeria spp. and antibiotic susceptibility of Listeria monocytogenes isolated from raw chicken and ready-to-eat chicken products in Jordan. Food Control 2011, 22, 586–590. [Google Scholar] [CrossRef]
- Saludes, M.; Troncoso, M.; Figueroa, G. Presence of Listeria monocytogenes in Chilean food matrices. Food Control 2015, 50, 331–335. [Google Scholar] [CrossRef]
- Santos Oliveira, T.; Milen Varjão, L.; Nunes da Silva, L.N.; de Castro Lisboa Pereira, R.; Hofer, E.; Vallim, D.C.; de Castro Almeida, R.C. Listeria monocytogenes at chicken slaughterhouse: Occurrence, genetic relationship among isolates and evaluation of antimicrobial susceptibility. Food Control 2018, 88, 131–138. [Google Scholar] [CrossRef]
- Sugiri, Y.D.; Gölz, G.; Meeyam, T.; Baumann, M.P.O.; Kleer, J.; Chaisowwong, W.; Alter, T. Prevalence and antimicrobial susceptibility of Listeria monocytogenes on chicken carcasses in Bandung, Indonesia. J. Food Prot. 2014, 77, 1407–1410. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Shu, Y.; Xia, W.; Xu, R.; Chen, Y. Modified PMA-qPCR method for rapid quantification of viable Lactobacillus spp. in fermented dairy products. Food Anal. Methods 2021, 14, 1908–1918. [Google Scholar] [CrossRef]
- Rantsiou, K.; Alessandria, V.; Urso, R.; Dolci, P.; Cocolin, L. Detection, quantification and vitality of Listeria monocytogenes in food as determined by quantitative PCR. Int. J. Food Microbiol. 2008, 121, 99–105. [Google Scholar] [CrossRef]
- Rørvik, L.M.; Yndestad, M. Listeria monocytogenes in foods in Norway. Int. J. Food Microbiol. 1991, 13, 97–104. [Google Scholar] [CrossRef]
- Kragh, M.L.; Thykier, M.; Truelstrup Hansen, L. A long-amplicon quantitative PCR assay with propidium monoazide to enumerate viable Listeria monocytogenes after heat and desiccation treatments. Food Microbiol. 2020, 86, 103310. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Tian, J.; Wang, B.; Wang, L. PMA based real-time fluorescent LAMP for detection of Vibrio parahaemolyticus in viable but nonculturable state. Food Control 2016, 63, 230–238. [Google Scholar] [CrossRef]
- Anwar, T.M.; Pan, H.; Chai, W.; Ed-Dra, A.; Fang, W.; Li, Y.; Yue, M. Genetic diversity, virulence factors, and antimicrobial resistance of Listeria monocytogenes from food, livestock, and clinical samples between 2002 and 2019 in China. Int. J. Food Microbiol. 2022, 366, 109572. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Pizarro-Cerda, J.; Lecuit, M. Invasion of mammalian cells by Listeria monocytogenes: Functional mimicry to subvert cellular functions. Trends Cell Biol. 2023, 13, 23–31. [Google Scholar] [CrossRef]
- Dramsi, S.; Dehoux, P.; Lebrun, M.; Goossens, P.L.; Cossart, P. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect. Immun. 1997, 65, 1615–1625. [Google Scholar] [CrossRef]
- Gaillard, J.L.; Berche, P.; Frehel, C.; Gouln, E.; Cossart, P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 1991, 65, 1127–1141. [Google Scholar] [CrossRef]
- Kocks, C.; Gouin, E.; Tabouret, M.; Berche, P.; Ohayon, H.; Cossart, P.L. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 1992, 68, 521–531. [Google Scholar] [CrossRef]
- de Souza Santos, M.; Orth, K. Subversion of the cytoskeleton by intracellular bacteria: Lessons from Listeria, Salmonella and Vibrio. Cell. Microbiol. 2015, 17, 164–173. [Google Scholar] [CrossRef]
- Furrer, B.; Candrian, U.; Hoefelein, C.; Luethy, J. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J. Appl. Bacteriol. 1991, 70, 372–379. [Google Scholar] [CrossRef]
- Portnoy, D.A.; Chakraborty, T.; Goebel, W.; Cossart, P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 1992, 60, 1263–1267. [Google Scholar] [CrossRef]
- Rabinovich, L.; Sigal, N.; Borovok, I.; Nir-Paz, R.; Herskovits, A.A. Prophage excision activates Listeria competence genes that promote phagosomal escape and virulence. Cell 2012, 150, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Kayode, A.J.; Okoh, A.I. Assessment of the molecular epidemiology and genetic multiplicity of Listeria monocytogenes recovered from ready-to-eat foods following the South African listeriosis outbreak. Sci. Rep. 2022, 12, 20129. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Camilo, R.; Magalhães, R.; Ferreira, V.; Santos, I.; Silva, J.; Almeida, G.; Teixeira, P. Biofilm formation among clinical and food isolates of Listeria monocytogenes. Int. J. Microbiol. 2013, 2013, 524975. [Google Scholar] [CrossRef]
- Harvey, J.; Keenan, K.P.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.B.; dos Santos, L.R.; Tagliari, V.Z.; Rizzo, N.N.; Trenhago, G.; de Oliveira, A.P.; Goetz, F.; do Nascimento, V.P. Quantification of biofilm production on polystyrene by Listeria, Escherichia coli and Staphylococcus aureus isolated from a poultry slaughterhouse. Braz. J. Microbiol. 2010, 41, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Chavant, P.; Martinie, B.; Meylheuc, T.; Hebraud, M. Listeria monocytogenes LO28: Surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 2002, 68, 728–737. [Google Scholar] [CrossRef]
- Guilbaud, M.; Piveteau, P.; Desvaux, M.; Brisse, S. Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Appl. Environ. Microbiol. 2014, 81, 1813. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Sert, D. Combined effect of benzalkonium chloride and ultrasound against Listeria monocytogenes biofilm on plastic surface. Lett. Appl. Microbiol. 2013, 57, 220–226. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; Carballo, J.; García-Fernández, C.; Capita, R.; Alonso-Calleja, C. Structure and viability of 24- and 72-h-old biofilms formed by four pathogenic bacteria on polystyrene and glass contact surfaces. Food Microbiol. 2018, 76, 513–517. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019, 82, 533–540. [Google Scholar] [CrossRef]
- Kadam, S.R.; Den Besten, H.M.W.; Van Der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Felices-Mercado, A.; García-Fernández, C.; Alonso-Calleja, C. Characterization of Listeria monocytogenes originating from the Spanish meat-processing chain. Foods 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- González-Gutiérrez, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Microbial load and antibiotic resistance in raw beef preparations from northwest Spain. Food Sci. Nutr. 2020, 8, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; McAllister, A.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016, 7, 68. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Doming, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Capita, R.; Álvarez-Fernández, E.; Fernández-Buelta, E.; Manteca, J.; Alonso-Calleja, C. Decontamination treatments can increase the prevalence of resistance to antibiotics of Escherichia coli naturally present on poultry. Food Microbiol. 2013, 34, 112–117. [Google Scholar] [CrossRef]
- Álvarez-Fernández, E.; Cancelo, A.; Díaz-Vega, C.; Capita, R.; Alonso-Calleja, C. Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: A comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Control 2013, 30, 227–234. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 5, 11–48. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef]
- Capita, R.; Vicente-Velasco, M.; Rodríguez-Melcón, C.; García-Fernández, C.; Carballo, J.; Alonso-Calleja, C. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci. Rep. 2019, 9, 15905. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Paludi, D.; Festino, A.R.; Ianieri, A. Listeria monocytogenes: Biofilms in food processing. Ital. J. Food Sci. 2014, 24, 203–213. [Google Scholar]
- Nourbakhsh, F.; Sadat, M.; Amineh, N.; Tajani, S.; Soheili, V. Bacterial biofilms and their resistance mechanisms: A brief look at treatment with natural agents. Folia Microbiol. 2022, 67, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, F.; Zhu, K.; Shen, J. Natural products that target virulence factors in antibiotic resistant Staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. The inhibitory effect of quercetin on biofilm formation of Listeria monocytogenes mixed culture and repression of virulence. Antioxidants 2022, 11, 1733. [Google Scholar] [CrossRef]









| Gene | Primer | Sequence (5′ → 3′) | Temp. (°C) | Product Size (pb) |
|---|---|---|---|---|
| lmo1030 | F | GCTTGTATTCACTTGGATTTGTCTGG | 62 | 509 |
| R | ACCATCCGCATATCTCAGCCAACT | |||
| lin0464 | F | CGCATTTATCGCCAAAACTC | 60 | 749 |
| R | TCGTGACATAGACGCGATTG | |||
| oxidoreductase | F | GCGGATAAAGGTGTTCGGGTCAA | 62 | 201 |
| R | ATTTGCTATCGTCCGAGGCTAGG | |||
| lmo0333 | F | GTACCTGCTGGGAGTACATA | 58 | 673 |
| R | CTGTCTCCATATCCGTACAG | |||
| namA | F | CGAATTCCTTATTCACTTGAGC | 58 | 463 |
| R | GGTGCTGCGAACTTAACTCA | |||
| prs | F | GCTGAAGAGATTGCGAAAGAAG | 58 | 370 |
| R | CAAAGAAACCTTGGATTTGCGG |
| Gene | Primer | Sequence (5′ → 3′) | Product Size (bp) | Concentration (µM) |
|---|---|---|---|---|
| hlyA | F | CCTAAGACGCCAATCGAA | 702 | 0.50 each |
| R | AAGCGCTTGCAACTGCTC | |||
| actA | F | GCTGATTTAAGAGATAGAGGAACA | 827 | 0.50 each |
| R | TTTATGTGGTTATTTGCTGTC | |||
| inlB | F | CTGGAAAGTTTGTATTTGGGAAA | 343 | 0.50 each |
| R | TTTCATAATCGCCATCATCACT | |||
| inlA | F | ACGAGTAACGGGACAAATGC | 800 | 0.25 each |
| R | CCCGACAGTGGTGCTAGATT | |||
| inlC | F | AATTCCCACAGGACACAACC | 517 | 0.20 each |
| R | CGGGAATGCAATTTTTCACTA | |||
| inlJ | F | TGTAACCCCCGCTTACACAGTT | 238 | 0.15 each |
| R | AGCGGCTTGGCAGTCTAATA | |||
| plcA | F | CTGCTTGAGCGTTCATGTCTCATCCCCC | 1484 | 0.20 each |
| R | CATGGGTTTCACTCTCCTTCTAC | |||
| prfA | F | CTGTTGGAGCTCTTCTTGGTGAAGCAATCG | 1060 | 0.20 each |
| R | AGCAACCTCGGTACCATATACTAACTC | |||
| iap | F | ACAAGCTGCACCTGTTGCAG | 131 | 0.50 each |
| R | TGACAGCGTGTGTAGTAGCA |
| Programs | Denaturation | Cycles | Elongation | |||
|---|---|---|---|---|---|---|
| hlyA | 94 °C/5 min | 30× | 94 °C/30 s | 50 °C/45 s | 72 °C/90 s | 72 °C/5 min |
| actA | 94 °C/2 min | 40× | 94 °C/180 s | 53 °C/60 s | 72 °C/120 s | 72 °C/5 min |
| inlB | 94 °C/2 min | 35× | 94 °C/45 s | 60 °C/45 s | 72 °C/90 s | 72 °C/8 min |
| inlA, inlC e inlJ | 94 °C/2 min | 30× | 94 °C/20 s | 55 °C/20 s | 72 °C/50 s | 72 °C/2 min |
| plcA y prfA | 95 °C/2 min | 35× | 95 °C/15 s | 60 °C/30 s | 72 °C/90 s | 72 °C/10 min |
| iap | 95 °C/2 min | 35× | 95 °C/15 s | 60 °C/30 s | 72 °C/90 s | 72 °C/10 min |
| Total Cells (without PMA) | Viable Cells (with PMA) | %Viable Cells | OCLA&PCR | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Ct | ng DNA | Log10 cfu/g | Ct | ng DNA | Log10 cfu/g | ||
| Breast1 | 39.24 | 0.000006 | 2.40 | >40 | <0.000003 | <2.15 | 56.4 | + |
| Wing1 | 38.98 | 0.000007 | 2.49 | >40 | <0.000003 | <2.15 | 46.3 | + |
| Breast2 | 38.70 | 0.000008 | 2.58 | >40 | <0.000003 | <2.15 | 37.5 | + |
| Thigh1 | 38.65 | 0.000009 | 2.60 | >40 | <0.000003 | <2.15 | 36.1 | + |
| Wing2 | 38.62 | 0.000009 | 2.61 | >40 | <0.000003 | <2.15 | 35.3 | + |
| Breast3 | 38.60 | 0.000009 | 2.61 | >40 | <0.000003 | <2.15 | 34.8 | − |
| Thigh2 | 38.04 | 0.000014 | 2.80 | >40 | <0.000003 | <2.15 | 22.8 | − |
| Wing3 | 37.89 | 0.000015 | 2.85 | >40 | <0.000003 | <2.15 | 20.4 | − |
| Wing4 | 37.67 | 0.000018 | 2.92 | >40 | <0.000003 | <2.15 | 17.3 | + |
| Breast4 | 36.14 | 0.000058 | 3.42 | >40 | <0.000003 | <2.15 | 5.4 | + |
| Drumstick1 | 36.13 | 0.000058 | 3.42 | >40 | <0.000003 | <2.15 | 5.4 | + |
| Wing5 | 37.44 | 0.000022 | 2.99 | 39.99 | 0.000003 | 2.16 | 14.6 | − |
| Drumstick2 | 37.87 | 0.000016 | 2.85 | 39.95 | 0.000003 | 2.17 | 20.8 | + |
| Wing6 | 37.67 | 0.000018 | 2.92 | 39.66 | 0.000004 | 2.27 | 22.3 | + |
| Wing7 | 38.61 | 0.000009 | 2.61 | 38.89 | 0.000007 | 2.52 | 81.0 | − |
| Drumstick3 | 33.12 | 0.000567 | 4.41 | 38.09 | 0.000013 | 2.78 | 2.3 | + |
| Drumstick4 | 34.63 | 0.000181 | 3.91 | 37.44 | 0.000022 | 2.99 | 12.0 | + |
| Drumstick5 | 34.50 | 0.000199 | 3.96 | 36.36 | 0.000049 | 3.35 | 24.6 | + |
| Drumstick6 | 35.23 | 0.000115 | 3.72 | 36.11 | 0.000059 | 3.43 | 51.7 | + |
| Drumstick7 | 30.66 | 0.003628 | 5.22 | 34.57 | 0.000190 | 3.93 | 5.2 | + |
| Pattern (Number of Isolates) | Antibiotic | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | OX | FOX | CTX | FEP | CN | E | VA | SXT | RD | TE | C | CIP | ENR | F | |
| 1 (19) | S | R | R | R | R | S | S | S | S | S/I * | S | S | I | S/I * | S/I * |
| 2 (1) | S | R | R | R | R | S | S | S | R | I | S | S | I | I | I |
| 3 (6) | S | R | R | R | R | S | S | S | S | R | S | S | I | S/I * | S/I * |
| 4 (3) | S | R | R | R | R | S | S | S | S | S/I * | S | S | R | S/I * | S/I * |
| 5 (1) | S | R | R | R | R | S | S | S | S | S | S | S | I | R | S |
| 6 (2) | S | R | R | R | R | S | S | S | S | S | S | S | I | S/I * | R |
| 7 (1) | S | R | R | R | R | S | R | S | S | I | S | S | I | I | R |
| 8 (1) | S | R | R | R | R | S | S | S | S | R | S | S | R | I | I |
| 9 (1) | S | R | R | R | R | S | S | S | R | R | S | S | I | I | I |
| 10 (1) | S | R | R | R | R | S | S | S | S | R | S | S | I | S | R |
| 11 (2) | S | R | R | R | R | S | S | S | R | S/I * | S | S | I | I | R |
| 12 (1) | S | R | R | R | R | S | S | S | R | I | S | S | R | I | R |
| 13 (1) | S | R | R | R | R | S | R | S | R | R | I | S | I | I | S |
| 14 (2) | S | R | R | R | R | S | S | S | R | R | S | S | R | I | S |
| 15 (1) | S | R | R | R | R | S | R | S | S | R | S | S | R | I | R |
| 16 (1) | S | R | R | R | R | S | R | S | R | R | S | S | I | I | R |
| 17 (1) | S | R | R | R | R | R | S | S | R | R | S | S | R | I | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panera-Martínez, S.; Capita, R.; García-Fernández, C.; Alonso-Calleja, C. Viability and Virulence of Listeria monocytogenes in Poultry. Microorganisms 2023, 11, 2232. https://doi.org/10.3390/microorganisms11092232
Panera-Martínez S, Capita R, García-Fernández C, Alonso-Calleja C. Viability and Virulence of Listeria monocytogenes in Poultry. Microorganisms. 2023; 11(9):2232. https://doi.org/10.3390/microorganisms11092232
Chicago/Turabian StylePanera-Martínez, Sarah, Rosa Capita, Camino García-Fernández, and Carlos Alonso-Calleja. 2023. "Viability and Virulence of Listeria monocytogenes in Poultry" Microorganisms 11, no. 9: 2232. https://doi.org/10.3390/microorganisms11092232