Analysis of the Metabolic Response of Planktonic Cells and Biofilms of Klebsiella pneumoniae to Sublethal Disinfection with Sodium Hypochlorite Measured by NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Reactivation
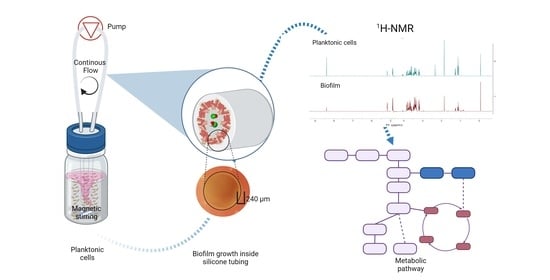
2.2. Culture of Biofilm and Planktonic Cells
2.3. Minimum Inhibitory Concentration of the Disinfectant in Planktonic Cells
2.4. Determination of the Minimum Biocidal Concentration
2.5. Stress Conditions
2.6. Exposure to Sub-Lethal Concentration of Sodium Hypochlorite
2.7. 1H-NMR Metabolic Profiling and Data Analysis
2.8. Putative Metabolite Identification and Metabolic Pathways and Statistical Analysis
3. Results
3.1. Determination of Sub-Lethal Concentration of Sodium Hypochlorite Disinfection and MBC
3.2. Concentration of Total and Free Chlorine during Stress
3.3. Metabolic Changes in Planktonic Cells and Biofilms
4. Discussion
4.1. Planktonic Cells Damage
4.2. Biofilm Defense Strategy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; WHO/UNICEF Joint Monitoring Programme for Water Supply, Sanitation and Hygiene (JMP); World Health Organization (WHO): Geneva, Switzerland; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2021; ISBN 978-92-4-003084-8. [Google Scholar]
- Ghordouei Milan, E.; Mahvi, A.H.; Nabizadeh, R.; Alimohammadi, M. What Is the Effect on Antibiotic Resistant Genes of Chlorine Disinfection in Drinking Water Supply Systems? A Systematic Review Protocol. Environ. Evid. 2022, 11, 11. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, Monitoring, and Controlling Biofilm Growth in Drinking Water Distribution Systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef]
- Lee, D.; Calendo, G.; Kopec, K.; Henry, R.; Coutts, S.; McCarthy, D.; Murphy, H.M. The Impact of Pipe Material on the Diversity of Microbial Communities in Drinking Water Distribution Systems. Front. Microbiol. 2021, 12, 779016. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Miller, S.E.; Kantor, R.S.; Nelson, K.L. Effect of Disinfectant Residual, PH, and Temperature on Microbial Abundance in Disinfected Drinking Water Distribution Systems. Environ. Sci. Water Res. Technol. 2021, 7, 78–92. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Goller, C.C.; Romeo, T. Environmental Influences on Biofilm Development. Curr. Top. Microbiol. Immunol. 2008, 322, 37–66. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia Coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella Pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 555. [Google Scholar] [CrossRef]
- Liu, K.; Tan, S.; Ye, W.; Hou, L.; Fang, B. Low-Concentration Iron Promotes Klebsiella Pneumoniae Biofilm Formation by Suppressing Succinic Acid. BMC Microbiol. 2022, 22, 95. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 1–15. [Google Scholar] [CrossRef]
- Gjersing, E.L.; Herberg, J.L.; Horn, J.; Schaldach, C.M.; Maxwell, R.S. NMR Metabolomics of Planktonic and Biofilm Modes of Growth in Pseudomonas Aeruginosa. Anal. Chem. 2007, 79, 8037–8045. [Google Scholar] [CrossRef]
- Leggett, A.; Li, D.-W.; Bruschweiler-Li, L.; Sullivan, A.; Stoodley, P.; Brüschweiler, R. Differential Metabolism between Biofilm and Suspended Pseudomonas Aeruginosa Cultures in Bovine Synovial Fluid by 2D NMR-Based Metabolomics. Biorxiv 2022. [Google Scholar]
- Booth, S.C.; Workentine, M.L.; Wen, J.; Shaykhutdinov, R.; Vogel, H.J.; Ceri, H.; Turner, R.J.; Weljie, A.M. Differences in Metabolism between the Biofilm and Planktonic Response to Metal Stress. J. Proteome Res. 2011, 10, 3190–3199. [Google Scholar] [CrossRef]
- Workentine, M.L.; Harrison, J.J.; Weljie, A.M.; Tran, V.A.; Stenroos, P.U.; Tremaroli, V.; Vogel, H.J.; Ceri, H.; Turner, R.J. Phenotypic and Metabolic Profiling of Colony Morphology Variants Evolved from Pseudomonas Fluorescens Biofilms. Environ. Microbiol. 2010, 12, 1565–1577. [Google Scholar]
- Ammons, M.C.B.; Tripet, B.P.; Carlson, R.P.; Kirker, K.R.; Gross, M.A.; Stanisich, J.J.; Copié, V. Quantitative NMR Metabolite Profiling of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Aureus Discriminates between Biofilm and Planktonic Phenotypes. J. Proteome Res. 2014, 13, 2973–2985. [Google Scholar] [CrossRef] [Green Version]
- Stipetic, L.H.; Dalby, M.J.; Davies, R.L.; Morton, F.R.; Ramage, G.; Burgess, K.E.V. A Novel Metabolomic Approach Used for the Comparison of Staphylococcus Aureus Planktonic Cells and Biofilm Samples. Metabolomics 2016, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Que, Y.; Wu, X.; Guan, T.; Guo, H. Metabolomics Deciphered Metabolic Reprogramming Required for Biofilm Formation. Sci. Rep. 2019, 9, 13160. [Google Scholar] [CrossRef] [Green Version]
- Alvear-Daza, J.J.; García-Barco, A.; Osorio-Vargas, P.; Gutiérrez-Zapata, H.M.; Sanabria, J.; Rengifo-Herrera, J.A. Resistance and Induction of Viable but Non Culturable States (VBNC) during Inactivation of E. coli and Klebsiella Pneumoniae by Addition of H2O2 to Natural Well Water under Simulated Solar Irradiation. Water Res. 2021, 188, 116499. [Google Scholar] [CrossRef]
- Zhang, B.; Powers, R. Analysis of Bacterial Biofilms Using NMR-Based Metabolomics. Future Med. Chem. 2012, 4, 1273–1306. [Google Scholar] [CrossRef] [Green Version]
- Drazic, A.; Kutzner, E.; Winter, J.; Eisenreich, W. Metabolic Response of Escherichia coli upon Treatment with Hypochlorite at Sub-Lethal Concentrations. PLoS ONE 2015, 10, e0125823. [Google Scholar] [CrossRef] [Green Version]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Kobayashi, S.D.; DeLeo, F.R. Klebsiella Pneumoniae Capsule Polysaccharide as a Target for Therapeutics and Vaccines. Comput. Struct. Biotechnol. J. 2019, 17, 1360–1366. [Google Scholar] [CrossRef]
- Sanabria, J.; Wist, J.; Pulgarin, C. Photocatalytic disinfection treatments: Viability, cultivability and metabolic changes of E. coli using diferent mesurements methods. Dyna 2011, 78, 150–157. [Google Scholar]
- Lu, M.; Park, C.; Lee, S.; Kim, B.; Oh, M.-K.; Um, Y.; Kim, J.; Lee, J. The Regulation of 2,3-Butanediol Synthesis in Klebsiella Pneumoniae as Revealed by Gene over-Expressions and Metabolic Flux Analysis. Bioprocess Biosyst. Eng. 2014, 37, 343–353. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative Stress in Bacteria and Protein Damage by Reactive Oxygen Species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar]
- Lushchak, V.I. Adaptive Response to Oxidative Stress: Bacteria, Fungi, Plants and Animals. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive Oxygen Species in Pathogen Clearance: The Killing Mechanisms, the Adaption Response, and the Side Effects. Front. Microbiol. 2020, 11, 622534. [Google Scholar] [CrossRef]
- Chen, X.; Stewart, P.S. Chlorine Penetration into Artificial Biofilm Is Limited by a Reaction−Diffusion Interaction. Environ. Sci. Amp Technol. 1996, 30, 2078–2083. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Chen, M.; Zhang, X.; Tang, C.Y.; Wu, Z. Acute Responses of Microorganisms from Membrane Bioreactors in the Presence of NaOCl: Protective Mechanisms of Extracellular Polymeric Substances. Environ. Sci. Technol. 2017, 51, 3233–3241. [Google Scholar] [CrossRef]
- Stewart, P.S.; Rayner, J.; Roe, F.; Rees, W.M. Biofilm Penetration and Disinfection Efficacy of Alkaline Hypochlorite and Chlorosulfamates. J. Appl. Microbiol. 2001, 91, 525–532. [Google Scholar] [CrossRef]







| Metabolite (mM) (±Standard Deviation) | Biofilm Control (without NaOCl) | Biofilm Treatment (NaOCl) | Planktonic Control (without NaOCl) | Planktonic Treatment (NaOCl) |
|---|---|---|---|---|
| Ethanol | 1.23 (0.79) | 2.15 (1.14) | 3.94 (0.21) | 3.09 (0.44) |
| Lactate | 1.38 (0.72) | 1.29 (0.17) | 1.48 (0.11) | 0.61 (0.09) |
| Acetate | 1.80 (0.35) | 1.97 (0.33) | 3.91 (0.25) | 3.30 (0.48) |
| Succinate | 0.03 (0.03) | 0.03 (0.02) | 0.89 (0.06) | 0.77 (0.12) |
| Formate | 1.62 (0.49) | 1.86 (0.37) | 5.52 (0.39) | 4.49 (0.71) |
| Glucose | 1.12 (0.07) | 1.22 (0.17) | 0.82 (0.05) | 0.94 (0.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Mendez, D.F.; Rengifo Herrera, J.A.; Sanabria, J.; Wist, J. Analysis of the Metabolic Response of Planktonic Cells and Biofilms of Klebsiella pneumoniae to Sublethal Disinfection with Sodium Hypochlorite Measured by NMR. Microorganisms 2022, 10, 1323. https://doi.org/10.3390/microorganisms10071323
Garcia Mendez DF, Rengifo Herrera JA, Sanabria J, Wist J. Analysis of the Metabolic Response of Planktonic Cells and Biofilms of Klebsiella pneumoniae to Sublethal Disinfection with Sodium Hypochlorite Measured by NMR. Microorganisms. 2022; 10(7):1323. https://doi.org/10.3390/microorganisms10071323
Chicago/Turabian StyleGarcia Mendez, David Felipe, Julián Andrés Rengifo Herrera, Janeth Sanabria, and Julien Wist. 2022. "Analysis of the Metabolic Response of Planktonic Cells and Biofilms of Klebsiella pneumoniae to Sublethal Disinfection with Sodium Hypochlorite Measured by NMR" Microorganisms 10, no. 7: 1323. https://doi.org/10.3390/microorganisms10071323