Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function
Abstract
:1. Introduction
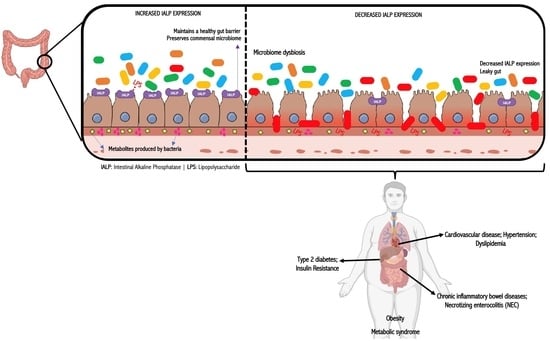
2. IALP and Intestinal Barrier Function
3. IALP and Inflammatory Bowel Disease
4. IALP and Necrotizing Enterocolitis (NEC)
5. IALP and Metabolic Dysfunction
6. IALP—How Can It Be Modulated?
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suzuki, U.Y.K.; Takashi, M. Uber ein enzyme–phytase‖ das anhydro-oxy-methylen-diphosphorsaure spaltet. Bull. Coll. Agric. Tokyo Imp. Univ. 1907, 7, 503–512. [Google Scholar]
- Tsai, L.C.; Hung, M.W.; Chen, Y.H.; Su, W.C.; Chang, G.G.; Chang, T.C. Expression and regulation of alkaline phosphatases in human breast cancer MCF-7 cells. Eur. J. Biochem. 2000, 267, 1330–1339. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Benham, F.; Cottell, D.C.; Franks, M.; Wilson, P.D. Alkaline phosphatase activity in human bladder tumor cell lines. J. Histochem. Cytochem. 1997, 25, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Mornet, E.; Stura, E.; Lia-Baldini, A.S.; Stigbrand, T.; Menez, A.; Le Du, M.H. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J. Biol. Chem. 2001, 276, 31171–31178. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.J.; Henthorn, P.S.; Lafferty, M.A.; Slaughter, C.; Raducha, M.; Harris, H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA 1986, 83, 7182–7186. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.; Singh, S.K.; Pal, D.; Khajuria, R.; Mandal, A.K.; Prasad, R. Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol. Cell. Biochem. 2012, 369, 287–293. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef]
- Chan, J.R.; Stinson, R.A. Dephosphorylation of phosphoproteins of human liver plasma membranes by endogenous and purified liver alkaline phosphatases. J. Biol. Chem. 1986, 261, 7635–7639. [Google Scholar] [CrossRef]
- Anagnostou, F.; Plas, C.; Forest, N. Ecto-alkaline phosphatase considered as levamisole-sensitive phosphohydrolase at physiological pH range during mineralization in cultured fetal calvaria cells. J. Cell. Biochem. 1996, 60, 484–494. [Google Scholar] [CrossRef]
- Scheibe, R.J.; Kuehl, H.; Krautwald, S.; Meissner, J.D.; Mueller, W.H. Ecto-alkaline phosphatase activity identified at physiological pH range on intact P19 and HL-60 cells is induced by retinoic acid. J. Cell. Biochem. 2000, 76, 420–436. [Google Scholar] [CrossRef]
- Nonoyama, S.; Karakida, T.; Chiba-Ohkuma, R.; Yamamoto, R.; Ujiie, Y.; Nagano, T.; Yamakoshi, Y.; Gomi, K. Development and Characterization of Alkaline Phosphatase-Positive Human Umbilical Cord Perivascular Cells. Cells 2021, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Palmer, G.; Bonjour, J.P.; Caverzasio, J. Regulation of Alkaline Phosphatase Activity by p38 MAP Kinase in Response to Activation of Gi Protein-Coupled Receptors by Epinephrine in Osteoblast-Like Cells. Endocrinology 1999, 140, 3177–3182. [Google Scholar] [CrossRef]
- Calhau, C.; Martel, F.; Hipólito-Reis, C.; Azevedo, I. Differences between duodenal and jejunal rat alkaline phosphatase. Clin. Biochem. 2000, 33, 571–577. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Alpers, D.H.; Akiba, Y.; Katayama, S.; Shinozaki, R.; Kaunitz, J.D.; Ohshima, S.; Akita, M.; Takahashi, S.; et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G207–G214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchet, R.; Millan, J.L.; Magne, D. Multisystemic functions of alkaline phosphatases. Methods Mol. Biol. 2013, 1053, 27–51. [Google Scholar]
- Geddes, K.; Philpott, D.J. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology 2008, 135, 8–12. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Meheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. 2003, 169, 169–176. [Google Scholar] [CrossRef]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Zähringer, U.; Rietschel, E.T.; Carroll, S.F.; Koch, M.H.; Kusumoto, S.; Seydel, U. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 1998, 161, 5464–5471. [Google Scholar]
- Galanos, C.; Lüderitz, O.; Rietschel, E.T.; Westphal, O.; Brade, H.; Brade, L.; Freudenberg, M.; Schade, U.; Imoto, M.; Yoshimura, H.; et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985, 148, 1–5. [Google Scholar] [CrossRef]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Ramasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef]
- Malo, M.S.; Moaven, O.; Muhammad, N.; Biswas, B.; Alam, S.N.; Economopoulos, K.P.; Gul, S.S.; Hamarneh, S.R.; Malo, N.S.; Teshager, A.; et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G826–G838. [Google Scholar] [CrossRef]
- Alam, S.N.; Yammine, H.; Moaven, O.; Ahmed, R.; Moss, A.K.; Biswas, B.; Muhammad, N.; Biswas, R.; Raychowdhury, A.; Kaliannan, K.; et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014, 259, 715–722. [Google Scholar] [CrossRef]
- Akiba, Y.; Mizumori, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1223–G1233. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Carr, A.; Corner, G.A.; Togel, L.; Davalos-Salas, M.; Tran, H.; Chueh, A.C.; Al-Obaidi, S.; Chionh, F.; Ahmed, N.; et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J. Biol. Chem. 2014, 289, 25306–25316. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Am. Coll. Surg. 2016, 222, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Larrick, J.W.; Mendelsohn, A.R. Supplementation with Brush Border Enzyme Alkaline Phosphatase Slows Aging. Rejuvenation Res. 2020, 23, 171–175. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Tuin, A.; Poelstra, K.; de Jager-Krikken, A.; Bok, L.; Raaben, W.; Velders, M.P.; Dijkstra, G. Role of alkaline phosphatase in colitis in man and rats. Gut 2009, 58, 379–387. [Google Scholar] [CrossRef]
- Torres, M.I.; Lorite, P.; Lopez-Casado, M.A.; Rios, A. A new approach using tissue alkaline phosphatase histochemistry to identify Crohn’s disease. Pathol. Res. Pract. 2007, 203, 485–487. [Google Scholar] [CrossRef]
- Ramasamy, S.; Nguyen, D.D.; Eston, M.A.; Alam, S.N.; Moss, A.K.; Ebrahimi, F.; Biswas, B.; Mostafa, G.; Chen, K.T.; Kaliannan, K.; et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm. Bowel Dis. 2011, 17, 532–542. [Google Scholar] [CrossRef]
- Lee, C.; Chun, J.; Hwang, S.W.; Kang, S.J.; Im, J.P.; Kim, J.S. The effect of intestinal alkaline phosphatase on intestinal epithelial cells, macrophages and chronic colitis in mice. Life Sci. 2014, 100, 118–124. [Google Scholar] [CrossRef]
- Martinez-Moya, P.; Ortega-Gonzalez, M.; Gonzalez, R.; Anzola, A.; Ocon, B.; Hernandez-Chirlaque, C.; Lopez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; et al. Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol. Res. 2012, 66, 144–153. [Google Scholar] [CrossRef]
- Danielak, A.; Wojcik, D.; Mazur-Bialy, A.; Surmiak, M.; Bilski, J.; Targosz, A.; Magierowski, M.; Chmura, A.; Strzalka, M.; Krzysiek-Maczka, G.; et al. Intestinal Alkaline Phosphatase Combined with Voluntary Physical Activity Alleviates Experimental Colitis in Obese Mice. Involvement of Oxidative Stress, Myokines, Adipokines and Proinflammatory Biomarkers. Antioxidants 2021, 10, 240. [Google Scholar] [CrossRef]
- Wojcik-Grzybek, D.; Hubalewska-Mazgaj, M.; Surmiak, M.; Sliwowski, Z.; Dobrut, A.; Mlodzinska, A.; Wojcik, A.; Kwiecien, S.; Magierowski, M.; Mazur-Bialy, A.; et al. The Combination of Intestinal Alkaline Phosphatase Treatment with Moderate Physical Activity Alleviates the Severity of Experimental Colitis in Obese Mice via Modulation of Gut Microbiota, Attenuation of Proinflammatory Cytokines, Oxidative Stress Biomarkers and DNA Oxidative Damage in Colonic Mucosa. Int. J. Mol. Sci. 2022, 23, 2964. [Google Scholar]
- Lukas, M.; Drastich, P.; Konecny, M.; Gionchetti, P.; Urban, O.; Cantoni, F.; Bortlik, M.; Duricova, D.; Bulitta, M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1180–1186. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef]
- Mankertz, J.; Amasheh, M.; Krug, S.M.; Fromm, A.; Amasheh, S.; Hillenbrand, B.; Tavalali, S.; Fromm, M.; Schulzke, J.D. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009, 336, 67–77. [Google Scholar] [CrossRef]
- Jager, S.; Stange, E.F.; Wehkamp, J. Inflammatory bowel disease: An impaired barrier disease. Langenbecks Arch. Surg. 2013, 398, 1–12. [Google Scholar] [CrossRef]
- Michielan, A.; D’Inca, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [Green Version]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef]
- Heath, M.; Buckley, R.; Gerber, Z.; Davis, P.; Linneman, L.; Gong, Q.; Barkemeyer, B.; Fang, Z.; Good, M.; Penn, D.; et al. Association of Intestinal Alkaline Phosphatase With Necrotizing Enterocolitis Among Premature Infants. JAMA Netw. Open 2019, 2, e1914996. [Google Scholar] [CrossRef]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K.; Canadian Neonatal, N. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [Green Version]
- Young, C.; Sharma, R.; Handfield, M.; Mai, V.; Neu, J. Biomarkers for infants at risk for necrotizing enterocolitis: Clues to prevention? Pediatr. Res. 2009, 65, 91–97. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Nanthakumar, N.; Meng, D.; Goldstein, A.M.; Zhu, W.; Lu, L.; Uauy, R.; Llanos, A.; Claud, E.C.; Walker, W.A. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE 2011, 6, e17776. [Google Scholar] [CrossRef] [Green Version]
- Heinzerling, N.P.; Liedel, J.L.; Welak, S.R.; Fredrich, K.; Biesterveld, B.E.; Pritchard, K.A., Jr.; Gourlay, D.M. Intestinal alkaline phosphatase is protective to the preterm rat pup intestine. J. Pediatr. Surg. 2014, 49, 954–960, discussion 960. [Google Scholar] [CrossRef] [Green Version]
- Mai, V.; Young, C.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Casella, G.; Theriaque, D.; Li, N.; Sharma, R.; Hudak, M.; et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 2011, 6, e20647. [Google Scholar] [CrossRef]
- Morais, J.; Marques, C.; Faria, A.; Teixeira, D.; Barreiros-Mota, I.; Durao, C.; Araujo, J.; Ismael, S.; Brito, S.; Cardoso, M.; et al. Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity. Nutrients 2021, 13, 1564. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart, A.; National Heart, L.; Blood, I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Narisawa, S.; Huang, L.; Iwasaki, A.; Hasegawa, H.; Alpers, D.H.; Millan, J.L. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 2003, 23, 7525–7530. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Shi, C.; Yang, J.; Zhang, P.; Ma, Y.; Wang, F.; Qin, H. Molecular regulation of the intestinal epithelial barrier: Implication in human diseases. Front. Biosci. 2011, 16, 2903–2909. [Google Scholar] [CrossRef] [Green Version]
- Kaliannan, K.; Hamarneh, S.R.; Economopoulos, K.P.; Nasrin Alam, S.; Moaven, O.; Patel, P.; Malo, N.S.; Ray, M.; Abtahi, S.M.; Muhammad, N.; et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 7003–7008. [Google Scholar] [CrossRef] [Green Version]
- Malo, M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. EBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Economopoulos, K.P.; Ward, N.L.; Phillips, C.D.; Teshager, A.; Patel, P.; Mohamed, M.M.; Hakimian, S.; Cox, S.B.; Ahmed, R.; Moaven, O.; et al. Prevention of antibiotic-associated metabolic syndrome in mice by intestinal alkaline phosphatase. Diabetes Obes. Metab. 2016, 18, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Gardener, H.; Rundek, T.; Markert, M.; Wright, C.B.; Elkind, M.S.; Sacco, R.L. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J. Gen. Intern. Med. 2012, 27, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [Green Version]
- Palmnas, M.S.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Burke, M.V.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, C.L.; Sikaroodi, M.; Lamb, E.; Shoemaker, S.; Gillevet, P.M. High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann. Epidemiol. 2015, 25, 736–742.e734. [Google Scholar] [CrossRef]
- Gul, S.S.; Hamilton, A.R.; Munoz, A.R.; Phupitakphol, T.; Liu, W.; Hyoju, S.K.; Economopoulos, K.P.; Morrison, S.; Hu, D.; Zhang, W.; et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl. Physiol. Nutr. Metab. 2017, 42, 77–83. [Google Scholar] [CrossRef]
- Ghosh, N.K.; Fishman, W.H. On the Mechanism of Inhibition of Intestinal Alkaline Phosphatase by l-Phenylalanine. J. Biol. Chem. 1966, 241, 2516–2522. [Google Scholar] [CrossRef]
- Lalles, J.P. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010, 68, 323–332. [Google Scholar] [CrossRef]
- Bamba, T.; Vaja, S.; Murphy, G.M.; Dowling, R.H. Effect of fasting and feeding on polyamines and related enzymes along the villus: Crypt axis. Digestion 1990, 46 (Suppl. S2), 424–429. [Google Scholar] [CrossRef]
- Antoniazzi, L.; Arroyo-Olivares, R.; Bittencourt, M.S.; Tada, M.T.; Lima, I.; Jannes, C.E.; Krieger, J.E.; Pereira, A.C.; Quintana-Navarro, G.; Muniz-Grijalvo, O.; et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2014–2022. [Google Scholar] [CrossRef]
- Ismael, S.; Silvestre, M.P.; Vasques, M.; Araujo, J.R.; Morais, J.; Duarte, M.I.; Pestana, D.; Faria, A.; Pereira-Leal, J.B.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Whitehead, R.H.; Young, G.P.; Bhathal, P.S. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut 1986, 27, 1457–1463. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.R.; Nov, R.; Fielding, M.; Mcintyre, A.; Finch, C.F.; Rosella, O.; Mariadason, J.M.; Barkla, D.H.; Young, G.P. Relationship of hydrolase activities to epithelial cell turnover in distal colonic mucosa of normal rats. J. Gastroenterol. Hepatol. 1999, 14, 866–872. [Google Scholar] [CrossRef]
- Stenson, W.F.; Seetharam, B.; Talkad, V.; Pickett, W.; Dudeja, P.; Brasitus, T.A. Effects of dietary fish oil supplementation on membrane fluidity and enzyme activity in rat small intestine. Biochem. J. 1989, 263, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.A.; Wang, H.; Hachey, D.L.; Shulman, R.J.; Perkinson, J.S.; Rosenberger, J.; Mersmann, H.J. Mersmann. Jejunal Brush Border Hydrolase Activity Is Higher in Tallow-Fed Pigs than in Corn Oil-Fed Pigs. J. Nutr. 1994, 124, 1996–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takase, S.; Goda, T. Effects of medium-chain triglycerides on brush border membrane-bound enzyme activity in rat small intestine. J. Nutr. 1990, 120, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Kaur, J.; Ojha, S.; Mahmood, A. Dietary fat effects on brush border membrane composition and enzyme activities in rat intestine. Ann. Nutr. Metab. 1996, 40, 269–276. [Google Scholar] [CrossRef]
- Kaur, J.; Madan, S.; Hamid, A.; Singla, A.; Mahmood, A. Intestinal alkaline phosphatase secretion in oil-fed rats. Dig. Dis. Sci. 2007, 52, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.M.; Zanetti, R.; Santa-María, C.; Ruíz-Gutiérrez, V. Effects of two highly monounsaturated oils on lipid composition and enzyme activities in rat jejunum. Biosci. Rep. 2000, 20, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Wahnon, R.; Cogan, U.; Mokady, S. Dietary fish oil modulates the alkaline phosphatase activity and not the fluidity of rat intestinal microvillus membrane. J. Nutr. 1992, 122, 1077–1084. [Google Scholar] [CrossRef]
- Ghafoorunissa, S.A.I. Influence of dietary partially hydrogenated fat high in trans fatty acids on lipid composition and function of intestinal brush border membrane in rats. J. Nutr. Biochem. 2001, 12, 116–120. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katayama, T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br. J. Nutr. 2019, 121, 146–154. [Google Scholar] [CrossRef]
- Bornet, F.R.; Brouns, F. Immune-stimulating and gut health-promoting properties of short-chain fructo-oligosaccharides. Nutr. Rev. 2002, 60 Pt 1, 326–334. [Google Scholar] [CrossRef]
- Watzl, B.; Girrbach, S.; Roller, M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005, 93 (Suppl. S1), S49–S55. [Google Scholar] [CrossRef]
- Tanabe, H.; Ito, H.; Sugiyama, K.; Kiriyama, S.; Morita, T. Dietary indigestible components exert different regional effects on luminal mucin secretion through their bulk-forming property and fermentability. Biosci. Biotechnol. Biochem. 2006, 70, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Komura, M.; Fukuta, T.; Genda, T.; Hino, S.; Aoe, S.; Kawagishi, H.; Morita, T. A short-term ingestion of fructo-oligosaccharides increases immunoglobulin A and mucin concentrations in the rat cecum, but the effects are attenuated with the prolonged ingestion. Biosci. Biotechnol. Biochem. 2014, 78, 1592–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Selma-Royo, M.; Garcia-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- Negrão, M.R.; Keating, E.; Faria, A.; Azevedo, I.; Martins, M.J. Acute effect of tea, wine, beer, and polyphenols on ecto-alkaline phosphatase activity in human vascular smooth muscle cells. J. Agric. Food Chem. 2006, 54, 4982–4988. [Google Scholar] [CrossRef]
- Zhou, Y.; Ruan, Z.; Zhou, L.; Yang, Y.; Mi, S.; Deng, Z.; Yin, Y. Chlorogenic acid decreased intestinal permeability and ameliorated intestinal injury in rats via amelioration of mitochondrial respiratory chain dysfunction. Food Sci. Biotechnol. 2016, 25, 253–260. [Google Scholar] [CrossRef]
- Lalles, J.P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- Lalles, J.P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef]
- Hamarneh, S.R.; Kim, B.M.; Kaliannan, K.; Morrison, S.A.; Tantillo, T.J.; Tao, Q.; Mohamed, M.M.R.; Ramirez, J.M.; Karas, A.; Liu, W.; et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig. Dis. Sci. 2017, 62, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.W.; Kim, J.H.; Lee, C.; Im, J.P.; Kim, J.S. Intestinal alkaline phosphatase ameliorates experimental colitis via toll-like receptor 4-dependent pathway. Eur. J. Pharmacol. 2018, 820, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Khailova, L.; Treece, A.; Robison, J.; Soranno, D.E.; Jaggers, J.; Ing, R.J.; Lawson, S.; Lujan, S.O. Alkaline Phosphatase Treatment of Acute Kidney Injury in an Infant Piglet Model of Cardiopulmonary Bypass with Deep Hypothermic Circulatory Arrest. Sci. Rep. 2019, 9, 14175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickkers, P.; Snellen, F.; Rogiers, P.; Bakker, J.; Jorens, P.; Meulenbelt, J.; Spapen, H.; Tulleken, J.E.; Lins, R.; Ramael, S.; et al. Clinical pharmacology of exogenously administered alkaline phosphatase. Eur. J. Clin. Pharmacol. 2009, 65, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.A.; Kabir, M.; Hossain, M.I.; Ahmed, E.; Siddique, A.; Rashid, H.; Mahfuz, M.; Mondal, D.; Ahmed, T.; Petri, W.A.; et al. Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS. PLoS ONE 2019, 14, e0220397. [Google Scholar] [CrossRef] [Green Version]
- Barboza, M.S., Jr.; Silva, T.M.J.; Guerrant, R.L.; Lima, A.A. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz. J. Med. Biol. Res. 1999, 32, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.I.; Nahar, B.; Hamadani, J.D.; Ahmed, T.; Roy, A.K.; Brown, K.H. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, F.; Andre, C.; Emery, Y.; Forichon, J.; Descos, L.; Minaire, Y. Assessment of the lactulose-mannitol test in Crohn’s disease. Gut 1988, 29, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.; Meddings, J.; Collins, S.M.; Investigators, W.E.L. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 2004, 20, 1317–1322. [Google Scholar] [CrossRef]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef]



| Aim of Study | Route of Administration | Treatment Effect | Animal Model | Ref. |
|---|---|---|---|---|
| Examine whether co-administration of IALP with antibiotics early in life have a preventive role against metabolic syndrome | Oral IALP (100 units/mL drinking water) supplementation ad libitumfor three intermittent 7-day cycles | Co-administration of IALP with AZT early in life prevents mice from susceptibility to the later development of HFD-induced obesity and MetS | C57BL/6 mice | Economopoulos et al. (2016) [62] |
| Investigate whether oral IALP supplementation protects against alcohol-induced liver disease | Oral IALP supplementation (200 U/mL) for 10 days | IALP treatment protected mice from alcohol-induced hepatotoxicity and steatosis | Female C57BL/6 mice | Hamarneh et al. (2017) [100] |
| Evaluate whether the protective effect of IALP on DSS-induced colitis is mediated by the Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) pathway | IALP (300 IU/day) via oral gavage for 7 days | Oral gavage administration of IALP significantly attenuated the severity of colitis via the TLR4/NF-κB pathway | WT C57BL/6 mice and TLR4-/- mice | Hwang et al. (2018) [101] |
| Determine if bovine intestinal ALP (BiAP) infusion prevents AKI | BiAP was administered by continuous infusion (25 U/kg/hr) via a femoral central venous catheter | BiAP infusion corrects serum and tissue ALP deficiency and may prevent AKI | Porcine model of early infant CPB/DHCA -induced AKI | Davidson et al. (2019) [102] |
| Evaluate the effect of IALP combined with moderate physical activity (voluntary wheel running) on the experimental colitis | IALP (200 U/day) was administered intragastrical for 12 weeks | Oral IALP treatment synergistically favored healing of intestinal inflammation, strengthened the antioxidant defense, and ameliorated the course of experimental colitis | SD- and HFD-fed C57BL/6 mice with experimental colitis induced by TNBS | Danielak et al. (2021) [37] |
| Evaluate the effect of IALP combined with moderate physical activity (voluntary wheel running) on experimental colitis | Oral IALP supplementation (200 U/day) in drinking water for 2 weeks | Administration of IALP combined with moderate physical activity significantly reduced gross and microscopic inflammatory response and oxidative stress markers | HFD female C57BL/6J mice with experimental colitis induced by TNBS | Wojcik-Grzybek Dagmara et al. (2022) [38] |
| Aim of Study | Study Design | Route of Administration | Treatment Effect | Sample Size/ Estimated Enrollment | Ref. |
|---|---|---|---|---|---|
| Evaluate the safety and preliminary efficacy of exogenous ALP administered to patients with UC | Interventionalallocation: N/A; intervention model: single group assignment; masking: none (open label); primary purpose: treatment | bIAP bolus 30,000 U/24 h for 7 consecutive days via a duodenal catheter | AP enzyme treatment was well tolerated and nonimmunogenic | 21 | M. Lukas et al. [39] (2010) |
| Evaluate the safety, pharmacokinetics, and pharmacodynamics of IV administration of exogenous ALP | Randomized; double-blind; placebo-controlled sequential protocols | Administered exogenous, 10 min IV infusions (three ascending doses) or 24–72 h continuous (132.5–200 U kg−1 24 h−1) IV | Exogenous AP administration in severe sepsis patients may play a renal protective role | 103 | P. Pickkers et al. [103] (2009) |
| Evaluate whether alkaline phosphatase injections can reduce acute inflammation in patients with rheumatoid arthritis | Interventional (clinical trial); non-randomized | s.c. injections of bovine intestinal Alkaline Phosphatase daily subcutaneous treatment with two injections of 2000 IU bIAP for three days | Ongoing study | 6 | NCT01416493 * |
| Evaluate the efficacy and safety of bovine intestinal alkaline phosphatase (bIAP) in reducing the pro-inflammatory post-surgical responses | Allocation: randomized; intervention model: parallel assignment; masking: quadruple (participant, care provider, investigator, outcomes assessor); primary purpose: prevention | bIAP bolus and 8 h infusion intravenous as a bolus of bIAP (1000 IU) just prior to surgery followed by a 40 IU/kg bIAP infusion during the first 8 h post-surgery | Ongoing study | 53 | NCT01144611 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.M.; Ismael, S.; Morais, J.; Araújo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. https://doi.org/10.3390/microorganisms10040746
Santos GM, Ismael S, Morais J, Araújo JR, Faria A, Calhau C, Marques C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms. 2022; 10(4):746. https://doi.org/10.3390/microorganisms10040746
Chicago/Turabian StyleSantos, Gilberto Maia, Shámila Ismael, Juliana Morais, João R. Araújo, Ana Faria, Conceição Calhau, and Cláudia Marques. 2022. "Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function" Microorganisms 10, no. 4: 746. https://doi.org/10.3390/microorganisms10040746