Bee Venom and Its Two Main Components—Melittin and Phospholipase A2—As Promising Antiviral Drug Candidates
Abstract
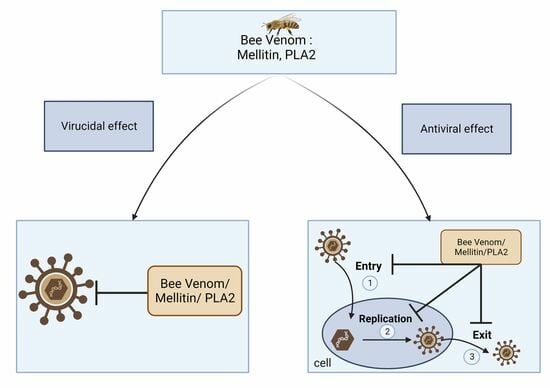
:1. Introduction
2. Effect of BV and MEL against Enveloped Viruses
2.1. Enveloped Viruses with Negative-Sense Single-Stranded RNA (ssRNA) Genome
2.2. Positive-Sense Single-Stranded RNA (ssRNA) Enveloped Viruses
2.3. Enveloped DNA Viruses
3. Effect of BV and MEL against Non-Enveloped Viruses
4. In Vitro Antiviral Effect of bvPLA2
5. Innovative Strategies Used to Reduce the Toxicity of BV and MEL
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 5–7. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; Von Georgi, R.; Münstedt, K. Apitherapy: Usage and Experience in German Beekeepers. Evid.-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef]
- Azam, N.K.; Ahmed, N.; Biswas, S.; Ara, N.; Rahman, M.; Hirashima, A.; Hasan, N. A Review on Bioactivities of Honey Bee Venom. Annu. Res. Rev. Biol. 2019, 30, 1–13. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First Characterization of the Venom from Apis Mellifera Syriaca, a Honeybee from the Middle East Region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.; Frangieh, J.; Rima, M.; Obeid, D.E.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee Venom in Cancer Therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Klupczynska, A.; Plewa, S.; Dereziński, P.; Garrett, T.J.; Rubio, V.Y.; Kokot, Z.J.; Matysiak, J. Identification and Quantification of Honeybee Venom Constituents by Multiplatform Metabolomics. Sci. Rep. 2020, 10, 21645. [Google Scholar] [CrossRef]
- Dempsey, C.E. The Actions of Melittin on Membranes. Biochim. Biophys. Acta 1990, 1031, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A Membrane-Active Peptide with Diverse Functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Kiesel, L.; Rabe, T.; Hauser, G.; Przylipiak, A.; Jadali, F.; Runnebaum, B. Stimulation of Luteinizing Hormone Release by Melittin and Phospholipase A2 in Rat Pituitary Cells. Mol. Cell. Endocrinol. 1987, 51, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carrasquer, G.; Li, M.; Yang, S.; Schwartz, M. Effect of Melittin on PD, Resistance and Short-Circuit Current in the Frog Gastric Mucosa. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1998, 1369, 346–354. [Google Scholar] [CrossRef]
- Hui, S.; Stewart, C.; Cherry, R.J. Electron Microscopic Observation of the Aggregation of Membrane Proteins in Human Erythrocyte by Melittin. Biochim. Biophys. Acta. 1990, 1023, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bitar, L.; Jundi, D.; Rima, M.; Sabatier, J.-M.; Fajloun, Z. Bee Venom PLA2 Versus Snake Venom PLA2: Evaluation of Structural and Functional Properties. Venoms Toxins 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A 2 Structure/Function, Mechanism, and Signaling. J. Lipid Res. 2009, 50, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Suzuki, A.; Sasaki, J.; Penninger, J. Phospholipase A2. J. Biochem. 2002, 131, 496–501. [Google Scholar]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Jiang, M.S. Possible Mechanisms of Action of Cobra Snake Venom Cardiotoxins and Bee Venom Melittin. Toxicon 1993, 31, 669–695. [Google Scholar] [CrossRef]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.M.; Coutard, B.; Fajloun, Z. The Cytotoxic Effect of Apis Mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and Pla2—On Colon Cancer Hct116 Cell Lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee Venom Therapy: Potential Mechanisms and Therapeutic Applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, S.S.; Kim, J.H.; Bae, C.S.; Choi, S.H. Inhibitory Effect of Whole Bee Venom in Adjuvant-Induced Arthritis. In Vivo 2005, 19, 801–805. [Google Scholar] [PubMed]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of Bee Venom. Clin. Rev. Allergy Immunol. 2018, 3, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Ko, E.; Park, S.K.; Ko, S.; Jun, C.-Y.; Shin, M.-K.; Hong, M.-C.; Hyunsu, B. Bee Venom Modulates Murine Th1/Th2 Lineage Development. Int. Immunopharmacol. 2005, 5, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Hong, I.P.; Woo, S.O.; Chun, S.N.; Park, K.K.; Nicholls, Y.M.; Pak, S.C. The Beneficial Effects of Honeybee-Venom Serum on Facial Wrinkles in Humans. Clin. Interv. Aging 2015, 10, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Gu, H.M.; Jeon, M.J.; Han, S.M.; Pak, S.C.; Lee, C.K.; Park, I.S.; et al. Therapeutic Effects of Bee Venom and Its Major Component, Melittin, on Atopic Dermatitis in Vivo and in Vitro. Br. J. Pharmacol. 2018, 175, 4310–4324. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, U.S.; Lee, S.D.; Kim, K.H.; Chung, K.H.; Chang, Y.C.; Park, K.K.; Lee, Y.C.; Kim, C.H. Effect of Bee Venom on Aromatase Expression and Activity in Leukaemic FLG 29.1 and Primary Osteoblastic Cells. J. Ethnopharmacol. 2005, 99, 245–252. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Kim, J.H.; Yoon, J.H.; Lee, J.D.; Han, H.J.; Mar, W.C.; Beitz, A.J.; Lee, J.H. The Analgesic Efficacy of Bee Venom Acupuncture for Knee Osteoarthritis: A Comparative Study with Needle Acupuncture. Am. J. Chin. Med. 2001, 29, 187–199. [Google Scholar] [CrossRef]
- Lin, T.Y.; Hsieh, C.L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF-Kappa B in NSCLC Cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef]
- Hong, S.J.; Gyu, S.R.; Hyung, I.Y.; Chang, S.Y.; Hyeong, G.K.; Jang, M.H.; Kim, C.J.; Choe, B.K.; Chung, J.H. Bee Venom Induces Apoptosis through Caspase-3 Activation in Synovial Fibroblasts of Patients with Rheumatoid Arthritis. Toxicon 2005, 46, 39–45. [Google Scholar] [CrossRef]
- Hesham, E.-S.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis Mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Abou Nader, R.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef]
- Mesquita, I.; Estaquier, J. Viral Manipulation of the Host Metabolic Network. Exp. Suppl. 2018, 109, 377–401. [Google Scholar] [PubMed]
- Koonin, E.V.; Senkevich, T.G.; Dolja, V.V. The Ancient Virus World and Evolution of Cells. Biol. Direct 2006, 27, 29. [Google Scholar] [CrossRef]
- Lecoq, H. Découverte Du Premier Virus, Le Virus de La Mosaïque Du Tabac: 1892 Ou 1898? Comptes Rendus L’académie Sci.-Ser. III-Sci. Vie 2001, 324, 929–933. [Google Scholar] [CrossRef]
- Simmonds, P.; Aiewsakun, P. Virus Classification—Where Do You Draw the Line? Arch. Virol. 2018, 163, 2037–2046. [Google Scholar] [CrossRef]
- Baltimore, D. Expression of Animal Virus Genomes. Bacteriol. Rev. 1971, 35, 235–241. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human Viruses: Discovery and Emergence. Phil. Trans. R. Soc. B 2012, 367, 2864–2871. [Google Scholar] [CrossRef]
- Dhar, J.; Samanta, J.; Kochhar, R. Corona Virus Disease-19 Pandemic: The Gastroenterologists’ Perspective. Indian J. Gastroenterol. 2020, 39, 220–231. [Google Scholar] [CrossRef]
- Word Health Organization. Vaccine Efficacy, Effectiveness and Protection. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 16 October 2023).
- Lahariya, C. Vaccine Epidemiology: A Review. J. Fam. Med. Prim. Care 2016, 5, 7. [Google Scholar] [CrossRef]
- Manoj, K.; Dipayan, R.; Purvi, P.; Manu, G.; Puneet, S. Viricidal Treatments for Prevention of Coronavirus Infection. Pathog. Glob. Health 2020, 114, 349–359. [Google Scholar]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and Strategies to Combat Viral Infections: A Review on FDA Approved Antiviral Drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Terrell, J.R.; Ashlyn, M.S. Nucleocapsid Structure of Negative Strand RNA Virus. Viruses 2020, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Control of Nonsegmented Negative-Strand RNA Virus Replication by SiRNA. Virus Res. 2004, 102, 27–35. [Google Scholar] [CrossRef]
- Cosset, F.-L.; Lavillette, D. Cell Entry of Enveloped Viruses. Adv. Genet. 2011, 73, 121–183. [Google Scholar] [CrossRef]
- Borchers, A.; Chang, C.; Gershwin, M.; Gershwin, L. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Girard, M.; Tam, J.; Assossou, O.; Kieny, M.P. The 2009 A (H1N1) Influenza Virus Pandemic: A Review. Vaccine 2010, 28, 4895–4902. [Google Scholar] [CrossRef]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.G.; Lee, J.S.; Kim, C.J. Inhibitory Effects of Bee Venom and Its Components against Viruses in Vitro and in Vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Brown, J.C.; Newcomb, W.W. Chapter 14 Rhabdoviridae. Perspect. Med. Virol. 1987, 3, 199–212. [Google Scholar] [CrossRef]
- Lichty, B.D.; Power, A.T.; Stojdl, D.F.; Bell, J.C. Vesicular Stomatitis Virus: Re-Inventing the Bullet. Trends Mol. Med. 2004, 10, 210–216. [Google Scholar] [CrossRef]
- Mantlo, E.; Bukreyeva, N.; Maruyama, J.; Paessler, S.; Huang, C. Antiviral Activities of Type I Interferons to SARS-CoV-2 Infection. Antivir. Res. 2020, 179, 104811. [Google Scholar] [CrossRef]
- Deonarain, R.; Alcamí, A.; Alexiou, M.; Dallman, M.J.; Gewert, D.R.; Porter, A.C.G. Impaired Antiviral Response and Alpha/Beta Interferon Induction in Mice Lacking Beta Interferon. J. Virol. 2000, 74, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M. Viruses and Interferon: A Fight for Supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z.; Zhou, D.; Chen, Y.; Hong, W.; Cao, L.; Yang, J.; Zhang, Y.; Shi, W.; Cao, Z.; et al. Virucidal Activity of a Scorpion Venom Peptide Variant Mucroporin-M1 against Measles, SARS-CoV and Influenza H5N1 Viruses. Peptides 2011, 32, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, W.F.; Musso, G.F.; Lieber, M.; Kaiser, E.T.; Kézdy, F.J. Kinetics and Mechanism of Hemolysis Induced by Melittin and by a Synthetic Melittin Analogue. Biophys. J. 1982, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Tosteson, M.T.; Holmes, S.J.; Razin, M.; Tosteson, D.C. Melittin Lysis of Red Cells. J. Membr. Biol. 1985, 87, 35–44. [Google Scholar] [CrossRef]
- Dufourcq, J.; Faucon, J.-F.; Fourche, G.; Dasseux, J.-L.; Le Maire, M.; Gulik-Krzywicki, T. Morphological Changes of Phosphatidylcholine Bilayers Induced by Melittin: Vesicularization, Fusion, Discoidal Particles. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1986, 859, 33–48. [Google Scholar] [CrossRef]
- Enria, D.A.; Briggiler, A.M.; Sánchez, Z. Treatment of Argentine Hemorrhagic Fever. Antivir. Res. 2008, 78, 132–139. [Google Scholar] [CrossRef]
- Albiol Matanic, V.; Castilla, V. Antiviral Activity of Antimicrobial Cationic Peptides against Junin Virus and Herpes Simplex Virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- McIntyre, W.; Netzband, R.; Bonenfant, G.; Biegel, J.; Miller, C.; Fuchs, G.; Henderson, E.; Arra, M.; Canki, M.; Fabris, D.; et al. Positive-Sense RNA Viruses Reveal the Complexity and Dynamics of the Cellular and Viral Epitranscriptomes during Infection. Nucleic Acids Res. 2018, 46, 5776–5791. [Google Scholar] [CrossRef]
- Wang, Y.; Grunewald, M.; Perlman, S. Coronaviruses: An Updated Overview of Their Replication and Pathogenesis. Methods Mol. Biol. 2020, 2203, 1–29. [Google Scholar] [CrossRef]
- Ramadan, R.H.; Mohamed Aly, F.; Abd EL-Daim, M. Evaluation of Antiviral Activity of Honeybee Venom on DNA and RNA Virus Models. Egypt. Acad. J. Biolog. Sci. 2009, 2, 247–258. [Google Scholar] [CrossRef]
- Sarhan, M.; El-Bitar, A.M.H.; Hotta, H. Potent Virucidal Activity of Honeybee “Apis Mellifera” Venom against Hepatitis C Virus. Toxicon 2020, 188, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Soman, N.; Lanza, G.; Heuser, J.; Schlesinger, P.; Wickline, S. Synthesis and Characterization of Stable Fluorocarbon Nanostructures as Drug Delivery Vehicles for Cytolytic Peptides. Nano Lett. 2008, 8, 1131–1136. [Google Scholar] [CrossRef]
- Hood, J.L.; Jallouk, A.P.; Campbell, N.; Ratner, L.; Wickline, S.A. Cytolytic Nanoparticles Attenuate HIV-1 Infectivity. Antivir. Ther. 2013, 18, 95–103. [Google Scholar] [CrossRef]
- Wachinger, M.; Saermark, T.; Erfle, V. Influence of Amphipathic Peptides on the HIV-1 Production in Persistently Infected T Lymphoma Cells. FEBS Lett. 1992, 309, 235–241. [Google Scholar] [CrossRef]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; Von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial Peptides Melittin and Cecropin Inhibit Replication of Human Immunodeficiency Virus 1 by Suppressing Viral Gene Expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef]
- Scott, L.J. Sitagliptin: A Review in Type 2 Diabetes. Drugs 2017, 77, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabia, M.W.; Alhakamy, N.A.; Ahmed, O.A.A.; Eljaaly, K.; Aloafi, A.L.; Mostafa, A.; Asfour, H.Z.; Aldarmahi, A.A.; Darwish, K.M.; Ibrahim, T.S.; et al. Repurposing of Sitagliptin-Melittin Optimized Nanoformula against Sars-CoV-2: Antiviral Screening and Molecular Docking Studies. Pharmaceutics 2021, 13, 307. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes Simplex Virus Infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Baghian, A.; Kousoulas, K.G. Role of the Na+, K+ Pump in Herpes Simplex Type 1-Induced Cell Fusion: Melittin Causes Specific Reversion of Syncytial Mutants with the Syn1 Mutation to Syn+ (Wild-Type) Phenotype. Virology 1993, 196, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sorin, M.; Kuhn, J.; Stasiak, A.; Stehle, T. Structural Insight into Non-Enveloped Virus Binding to Glycosaminoglycan Receptors: A Review. Viruses 2021, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-H.; Kim, D.-S.; Cho, W.; Nam, J.-H. Coxsackievirus B3 Regulates T-Cell Infiltration into the Heart by Lymphocyte Function-Associated Antigen-1 Activation via the CAMP/Rap1 Axis. J. Gen. Virol. 2014, 95, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Goksugur, N.; Goksugur, S. Images in Clinical Medicine. Hand, Foot, and Mouth Disease. N. Engl. J. Med. 2010, 362, e49. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Cepeda, C.; Martínez-Valverde, A.; del Mar Sabater-Recolons, M.; Jané-Salas, E.; Marí-Roig, A.; López-López, J. A Literature Review and Case Report of Hand, Foot and Mouth Disease in an Immunocompetent Adult. BMC Res. Notes 2016, 9, 165. [Google Scholar] [CrossRef]
- Kim, Y.W.; Chaturvedi, P.K.; Chun, S.N.; Lee, Y.G.; Ahn, W.S. Honeybee Venom Possesses Anticancer and Antiviral Effects by Differential Inhibition of HPV E6 and E7 Expression on Cervical Cancer Cell Line. Oncol. Rep. 2015, 33, 1675–1682. [Google Scholar] [CrossRef]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K.; et al. Broad-Spectrum Antiviral Agents: Secreted Phospholipase A2 Targets Viral Envelope Lipid Bilayers Derived from the Endoplasmic Reticulum Membrane. Sci. Rep. 2017, 7, 15931. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.C.; Lazdunski, M.; Doglio, A. Secreted Phospholipases A2, a New Class of HIV Inhibitors That Block Virus Entry into Host Cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.; Doglio, A. A Peptide Derived from Bee Venom-Secreted Phospholipase A2 Inhibits Replication of T-Cell Tropic HIV-1 Strains via Interaction with the CXCR4 Chemokine Receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef]
- Muller, V.D.; Soares, R.O.; dos Santos, N.N., Jr.; Trabuco, A.C.; Cintra, A.C.; Figueiredo, L.T.; Caliri, A.; Sampaio, S.V.; Aquino, V.H. Phospholipase A2 Isolated from the Venom of Crotalus Durissus Terrificus Inactivates Dengue Virus and Other Enveloped Viruses by Disrupting the Viral Envelope. PLoS ONE 2014, 9, e112351. [Google Scholar] [CrossRef]
- Muller, V.D.; Russo, R.R.; Cintra, A.C.; Sartim, M.A.; Alves-Paiva Rde, M.; Figueiredo, L.T.; Sampaio, S.V.; Aquino, V.H. Crotoxin and Phospholipases A2 from Crotalus Durissus Terrificus Showed Antiviral Activity against Dengue and Yellow Fever Viruses. Toxicon 2012, 59, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.A.; Shimizu, J.F.; de Oliveira, D.M.; Martins, D.O.S.; Cardoso-Sousa, L.; Cintra, A.C.O.; Aquino, V.H.; Sampaio, S.V.; Nicolau-Junior, N.; Sabino-Silva, R.; et al. Chikungunya Virus Entry Is Strongly Inhibited by Phospholipase A2 Isolated from the Venom of Crotalus Durissus Terrificus. Sci. Rep. 2021, 11, 8717. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kim, Y.S.; Lee, K.-S.; Seo, H.-S.; Lee, C.-Y.; Kim, K.K. Detoxification of Bee Venom Increases Its Anti-Inflammatory Activity and Decreases Its Cytotoxicity and Allergenic Activity. Appl. Biochem. Biotechnol. 2021, 193, 4068–4082. [Google Scholar] [CrossRef]
- El-Didamony, S.E.; Amer, R.I.; El-Osaily, G.H. Formulation, Characterization and Cellular Toxicity Assessment of a Novel Bee-Venom Microsphere in Prostate Cancer Treatment. Sci. Rep. 2022, 12, 13213. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhu, W.; Yang, L.; Yang, Y.; Peng, J. Melittin-Based Nano-Delivery Systems for Cancer Therapy. Biomolecules 2022, 12, 118. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Chen, Z.; Shang, Z.; Li, Y.; Xu, W.; Mo, Y.; Wang, X.; Xu, D.; Li, S.; et al. Melittin Tryptophan Substitution with a Fluorescent Amino Acid Reveals the Structural Basis of Selective Antitumor Effect and Subcellular Localization in Tumor Cells. Toxins 2022, 14, 428. [Google Scholar] [CrossRef]
- Falco, A.; Barrajón-Catalán, E.; Menéndez-Gutiérrez, M.P.; Coll, J.; Micol, V.; Estepa, A. Melittin-Loaded Immunoliposomes against Viral Surface Proteins, a New Approach to Antiviral Therapy. Antivir. Res. 2013, 97, 218–221. [Google Scholar] [CrossRef] [PubMed]


| Viruses | EC50 | SI | Mechanism of Action | References | ||
|---|---|---|---|---|---|---|
| Bee venom’s antiviral effect | Enveloped viruses | Respiratory Syncytial Virus (RSV) | 1.17 µg/mL | 5.34 | Virucidal effect | [49] |
| Influenza A (H1N1) | 1.81 µg/mL | 4.61 | Virucidal effect | [49] | ||
| Vesicular Stomatitis Virus (VSV) | 0.5 µg/mL | 17.22 | Inhibition of virus replication after virus enters the cells. Stimulating type I IFN signaling. Virucidal effect | [49] | ||
| West Nile Virus (WNV) | Virucidal activity | [63] | ||||
| Human Hepatitis C Virus (HCV) | 0.05 ng/mL | 400,000 | Direct virucidal activity. Probability to have effect on the entry of the virus in the cells. | [64] | ||
| Herpes Simplex Virus (HSV) | 1.52 µg/mL | 4.69 | Virucidal effect | [49] | ||
| Non-enveloped viruses | Coxsackievirus B3 (CVB3) | 0.5 µg/mL | 17.96 | Virucidal effect | [49] | |
| Enterovirus (EV-71) | 0.49 µg/mL | 18.3 | Virucidal effect Decrease VP1 mRNA expression. | [49] | ||
| Human Papillomavirus (HPV) | Downregulation of E6/E7 protein of HPV | [77] | ||||
| Adenovirus type-7 | Virucidal activity | [63] |
| Viruses | EC50 | SI | Mechanism of Action | References | ||
|---|---|---|---|---|---|---|
| MEL’s antiviral effect | Enveloped viruses | Vesicular Stomatitis Virus (VSV) | 1.18 µg/mL | 5.27 | Virucidal activity | [49] |
| Influenza Virus (H1N1) | 1.15 µg/mL | 6.66 | In vivo, protect mice from lethal dose of H1N1(virucidal effect) | [49] | ||
| Human Respiratory Syncytial Virus (RSV) | 0.35 µg/mL | 14.34 | Virucidal effect | [49] | ||
| Junin Virus (JV) | Antiviral activity (mechanism not dermine) | [60] | ||||
| HIV | 0.9–1.5 µM |
| [66,67,68] | |||
| SARS-CoV-2 (SIT-MEL) | 8.43 µM | [70] | ||||
| Herpes Simplex Virus (HSV) | 0.5 µM | Inhibiting the attachment of HSV-1 into hot cells by inhibiting the Na+, K+ pump leading to the inhibition of the cell fusion. | [72] | |||
| Non-enveloped viruses | Enterovirus 71 (EV-71) | 0.76 µg/mL | 5.75 | Decreasing four times the mRNA expression levels of capsid protein VP1 in EV-71-infected cells compared to untreated cells. | [49] | |
| Coxsackievirus H3 | 0.99 µg/mL | 4.40 | Virucidal activity | [49] |
| bvPLA2 | svPLA2 | ||
|---|---|---|---|
| Viruses | Mode of Action | Viruses | Mode of Action |
| HIV-1 | Blocking the virus entry [79,80] | HCV |
|
| Dengue virus (DENV) | |||
| Japanese encephalititis virus (JEV) | |||
| YFV and DENV | Virucidal effect [82] | ||
| Rocio virus Oropouche virus Mayaro virus | Virucidal effect [81] | ||
| HCV, DENV, and JEV | Virucidal effect [78] | Chikungunya virus | Inhibition of viral entry into cells [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacoub, C.; Wehbe, R.; Roufayel, R.; Fajloun, Z.; Coutard, B. Bee Venom and Its Two Main Components—Melittin and Phospholipase A2—As Promising Antiviral Drug Candidates. Pathogens 2023, 12, 1354. https://doi.org/10.3390/pathogens12111354
Yaacoub C, Wehbe R, Roufayel R, Fajloun Z, Coutard B. Bee Venom and Its Two Main Components—Melittin and Phospholipase A2—As Promising Antiviral Drug Candidates. Pathogens. 2023; 12(11):1354. https://doi.org/10.3390/pathogens12111354
Chicago/Turabian StyleYaacoub, Carole, Rim Wehbe, Rabih Roufayel, Ziad Fajloun, and Bruno Coutard. 2023. "Bee Venom and Its Two Main Components—Melittin and Phospholipase A2—As Promising Antiviral Drug Candidates" Pathogens 12, no. 11: 1354. https://doi.org/10.3390/pathogens12111354