Multi-Dimensionality Immunophenotyping Analyses of MAIT Cells Expressing Th1/Th17 Cytokines and Cytotoxic Markers in Latent Tuberculosis Diabetes Comorbidity
Abstract
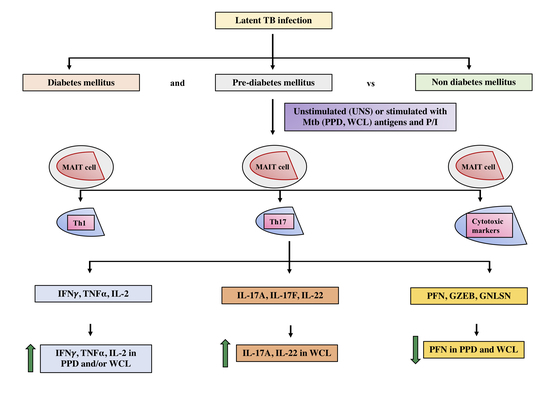
:1. Introduction
2. Methods
2.1. Study Ethics
2.2. Study Participants
2.3. Isolation and Thawing of Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Antigens and Stimulation
2.5. PBMC Staining and Flow Cytometry
2.6. Data Analysis
3. Results
3.1. UMAP Analysis of MAIT Cell Expressing Th1 Cytokines in LTB Comorbidities
3.2. Altered GM of MAIT Cells Expressing Th1 Cytokines
3.3. UMAP Analysis of MAIT Cell Expressing Th17 Cytokines in LTB Comorbidities
3.4. MAIT Cell Expressing Th17 Cytokines Were Not Significantly Different among LTB Comorbidities
3.5. UMAP Analysis of MAIT Cell Expressing Cytotoxic Markers in LTB Comorbidities
3.6. MAIT Cells Expressing Cytotoxic Markers Were Significantly Reduced in LTB-PDM Individuals
3.7. Correlation and PCA Analysis of MAIT Cells Expressing Th1, Th17 Cytokines and Cytotoxic Markers in LTB Coinfected Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, U.; Migliori, G.B.; Sester, M.; Rieder, H.L.; Ehlers, S.; Goletti, D.; Bossink, A.; Magdorf, K.; Hölscher, C.; Kampmann, B.; et al. LTBI: Latent tuberculosis infection or lasting immune responses to M.tuberculosis? A TBNET consensus statement. Eur. Respir. J. 2009, 33, 956–973. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Simmons, J.D.; Stein, C.M.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef]
- Getahun, H.; Matteelli, A.; Chaisson, R.E.; Raviglione, M. Latent Mycobacterium tuberculosis infection. N. Engl. J. Med. 2015, 372, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Comstock, G.W.; Livesay, V.T.; Woolpert, S.F. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 1974, 99, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Vynnycky, E.; Fine, P.E. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 1997, 119, 183–201. [Google Scholar] [CrossRef]
- Shea, K.M.; Kammerer, J.S.; Winston, C.A.; Navin, T.R.; Horsburgh, C.R., Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am. J. Epidemiol. 2014, 179, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lönnroth, K.; Roglic, G.; Harries, A.D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: From evidence to policy and practice. Lancet Diabetes Endocrinol. 2014, 2, 730–739. [Google Scholar] [CrossRef]
- Kumar, N.P.; Banurekha, V.V.; Nair, D.; Sridhar, R.; Kornfeld, H.; Nutman, T.B.; Babu, S. Coincident pre-diabetes is associated with dysregulated cytokine responses in pulmonary tuberculosis. PLoS ONE 2014, 9, e112108. [Google Scholar]
- Wallis, R.S.; Hafner, R. Advancing host-directed therapy for tuberculosis. Nat. Rev. Immunol. 2015, 15, 255–263. [Google Scholar] [CrossRef]
- Provine, N.M.; Klenerman, P. MAIT cells in health and disease. Annu. Rev. Immunol. 2020, 38, 203–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legoux, F.; Bellet, D.; Daviaud, C.; Morr, Y.E.; Darbois, A.; Niort, K.; Procopio, E.; Salou, M.; Gilet, J.; Ryffel, B.; et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 2019, 366, 494–499. [Google Scholar] [CrossRef]
- Viswanathan, V.; Kumpatla, S.; Aravindalochanan, V.; Rajan, R.; Chinnasamy, C.; Srinivasan, R.; Selvam, J.M.; Kapur, A. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS ONE 2012, 7, e41367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, R.; Schneider, M.; de la Harpe, S.M.; Harrop, T.W.R.; Hannaway, R.F.; Dearden, P.K.; Kirman, J.R.; Tyndall, J.D.A.; Vernall, A.J.; Ussher, J.E. TCR- or cytokine-activated CD8(+) mucosal- associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 2019, 28, 3061–3076. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.S.; Morgan, M.P.; Liuzzi, A.R.; Tyler, C.J.; Khan, M.W.; Szakmany, T.; Hall, J.E.; Moser, B.; Eberl, M. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J. Immunol. 2014, 193, 3704–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulouis, C.; Sia, W.R.; Gulam, M.Y.; Teo, J.Q.M.; Png, Y.T.; Phan, T.K.; Mak, J.Y.W.; Fairlie, D.P.; Poon, I.K.H.; Koh, T.H.; et al. Human MAIT cell cytolytic effector proteins synergize to overcome carbapenem resistance in Escherichia coli. PLoS Biol. 2020, 18, e3000644. [Google Scholar] [CrossRef] [PubMed]
- Meierovics, A.I.; Cowley, S.C. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J. Exp. Med. 2016, 213, 2793–2809. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.C.; Napier, R.J.; Lewinsohn, D.M. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol. Rev. 2015, 264, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, J.M.; Dash, P.; Roy, S.; McMurtrey, C.; Awad, W.; Reed, J.S.; Hammond, K.B.; Abdulhaqq, S.; Wu, H.L.; Burwitz, B.J.; et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 2017, 10, 802–813. [Google Scholar] [CrossRef] [Green Version]
- Saeidi, A.; Tien Tien, V.L.; Al-Batran, R.; Al-Darraji, H.A.; Tan, H.Y.; Yong, Y.K.; Ponnampalavanar, S.; Barathan, M.; Rukumani, D.V.; Ansari, A.W.; et al. Attrition of TCR Vα7.2+ CD161++ MAIT cells in HIV-tuberculosis co-infection is associated with elevated levels of PD-1 expression. PLoS ONE 2015, 10, e0124659. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Jin, H.M.; Cho, Y.N.; Kim, M.J.; Kang, J.H.; Jung, H.J.; Park, K.J.; Kee, H.J.; Kee, S.J.; Park, Y.W. Mucosal-associated invariant T cell deficiency in chronic obstructive pulmonary disease. COPD 2016, 13, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.B.; Akilimali, N.A.; Govender, P.; Sullivan, Z.A.; Cosgrove, C.; Pillay, M.; Lewinsohn, D.M.; Bishai, W.R.; Walker, B.D.; Ndung’u, T.; et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS ONE 2013, 8, e83474. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wang, X.; An, H.; Yang, B.; Cao, Z.; Liu, Y.; Su, J.; Zhai, F.; Wang, R.; Zhang, G.; et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am. J. Respir. Crit. Care Med. 2014, 190, 329–339. [Google Scholar] [PubMed]
- Paquin-Proulx, D.; Costa, P.R.; Terrassani Silveira, C.G.; Marmorato, M.P.; Cerqueira, N.B.; Sutton, M.S.; O’Connor, S.L.; Carvalho, K.I.; Nixon, D.F.; Kallas, E.G. Latent Mycobacterium tuberculosis infection is associated with a higher frequency of mucosal-associated invariant t and invariant natural killer T cells. Front. Immunol. 2018, 9, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, e152. [Google Scholar]
- Kumar, N.P.; Moideen, K.; Dolla, C.; Kumaran, P.; Babu, S. Prediabetes is associated with the modulation of antigen-specific Th1/Tc1 and Th17/Tc17 responses in latent Mycobacterium tuberculosis infection. PLoS ONE 2017, 12, e0178000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.P.; Moideen, K.; George, P.J.; Dolla, C.; Kumaran, P.; Babu, S. Coincident diabetes mellitus modulates Th1-, Th2-, and Th17-cell responses in latent tuberculosis in an IL-10- and TGF-beta-dependent manner. Eur. J. Immunol. 2016, 46, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.P.; Moideen, K.; George, P.J.; Dolla, C.; Kumaran, P.; Babu, S. Impaired cytokine but enhanced cytotoxic marker expression in Mycobacterium tuberculosis-induced CD8+ T cells in individuals with Type 2 Diabetes and latent Mycobacterium tuberculosis infection. J. Infect. Dis. 2016, 213, 866–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.P.; George, P.J.; Kumaran, P.; Dolla, C.; Kumaran, P.; Babu, S. Diminished systemic and antigen-specific type 1, type 17, and other proinflammatory cytokines in diabetic and prediabetic individuals with latent Mycobacterium tuberculosis infection. J. Infect Dis. 2014, 210, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- Hensel, R.L.; Kempker, R.R.; Tapia, J.; Oladele, A.; Blumberg, H.M.; Magee, M.J. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int. J. Tuberc. Lung Dis. 2016, 20, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Junior, J.L.; Gil-Santana, L.; Oliveira, C.A.; Castro, S.; Cafezeiro, A.S.; Daltro, C.; Netto, E.M.; Kornfeld, H.; Andrade, B.B. Glucose metabolism disorder is associated with pulmonary tuberculosis in individuals with respiratory symptoms from Brazil. PLoS ONE 2016, 11, e0153590. [Google Scholar] [CrossRef]
- Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009, 27, 393–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, C.L.; Abrahams, D.A.; Lerumo, L.; Janse van Rensburg, E.; Stone, L.; O’rie, T.; Pienaar, B.; de Kock, M.; Kaplan, G.; Mahomed, H.; et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 2011, 187, 2222–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, A.; Rozot, V.; Bellutti Enders, F.; Perreau, M.; Stalder, J.M.; Nicod, L.P.; Cavassini, M.; Calandra, T.; Blanchet, C.L.; Jaton, K.; et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat. Med. 2011, 17, 372–376. [Google Scholar] [CrossRef]
- Coulter, F.; Parrish, A.; Manning, D.; Kampmann, B.; Mendy, J.; Garand, M.; Lewinsohn, D.M.; Riley, E.M.; Sutherland, J.S. IL-17 Production from T Helper 17, Mucosal-associated invariant T and γδ cells in tuberculosis infection and disease. Front. Immunol. 2017, 8, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gherardin, N.A.; Souter, M.N.; Koay, H.F.; Mangas, K.M.; Seemann, T.; Stinear, T.P.; Eckle, S.B.; Berzins, S.P.; d’Udekem, Y.; Konstantinov, I.E.; et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 2018, 96, 507–525. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.J.; Yu, Y.Y.; et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Chen, X.; An, H.; Yang, B.; Zhang, F.; Cheng, X. Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling. Sci. Rep. 2016, 6, 32320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, R.; Monin, L.; Slight, S.; Uche, U.; Blanchard, E.; Fallert Junecko, B.A.; Ramos-Payan, R.; Stallings, C.L.; Reinhart, T.A.; Kolls, J.K.; et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014, 10, e1004099. [Google Scholar] [CrossRef]
- Hur, Y.G.; Gorak-Stolinska, P.; Ben-Smith, A.; Lalor, M.K.; Chaguluka, S.; Dacombe, R.; Doherty, T.M.; Ottenhoff, T.H.; Dockrell, H.M.; Crampin, A.C. Combination of cytokine responses indicative of latent TB and active TB in Malawian adults. PLoS ONE 2013, 8, e79742. [Google Scholar]
- Tang, X.Z.; Jo, J.; Tan, A.T.; Sandalova, E.; Chia, A.; Tan, K.C.; Lee, K.H.; Gehring, A.J.; De Libero, G.; Bertoletti, A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 2013, 190, 3142–3152. [Google Scholar] [CrossRef]
- Kathamuthu, G.R.; Kumar, N.P.; Moideen, K.; Menon, P.A.; Babu, S. Decreased frequencies of gamma/delta T cells expressing Th1/Th17 cytokine, cytotoxic, and immune markers in latent tuberculosis-diabetes/pre-diabetes comorbidity. Front. Cell. Infect. Microbiol. 2021, 11, 756854. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, L.; Caillat-Zucman, S.; Houdouin, V. Mucosal-associated invariant T cell interactions with commensal and pathogenic bacteria: Potential role in antimicrobial immunity in the child. Front. Immunol. 2017, 8, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeansyah, E.; Boulouis, C.; Kwa, A.L.H.; Sandberg, J.K. Emerging role for MAIT cells in control of antimicrobial resistance. Trends Microbiol. 2021, 29, 504–516. [Google Scholar] [CrossRef] [PubMed]








| Study Demographics | LTB-NDM | LTB-PDM | LTB DM |
|---|---|---|---|
| Number of subjects recruited (n) | 20 | 20 | 20 |
| Sex (M/F) | 10/10 | 11/9 | 11/9 |
| Median age in years (range) | 39.6 (24–62) | 45.9 (25–62) | 47.1 (25–60) |
| Glycated hemoglobin level, % | <5.48 (5.0–5.7) | >6.1 (5.7–6.3) | 8.43 (6.50–11.96) |
| QuantiFERON-TB gold assay | Positive | Positive | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathamuthu, G.R.; Pavan Kumar, N.; Moideen, K.; Dolla, C.; Kumaran, P.; Babu, S. Multi-Dimensionality Immunophenotyping Analyses of MAIT Cells Expressing Th1/Th17 Cytokines and Cytotoxic Markers in Latent Tuberculosis Diabetes Comorbidity. Pathogens 2022, 11, 87. https://doi.org/10.3390/pathogens11010087
Kathamuthu GR, Pavan Kumar N, Moideen K, Dolla C, Kumaran P, Babu S. Multi-Dimensionality Immunophenotyping Analyses of MAIT Cells Expressing Th1/Th17 Cytokines and Cytotoxic Markers in Latent Tuberculosis Diabetes Comorbidity. Pathogens. 2022; 11(1):87. https://doi.org/10.3390/pathogens11010087
Chicago/Turabian StyleKathamuthu, Gokul Raj, Nathella Pavan Kumar, Kadar Moideen, Chandrakumar Dolla, Paul Kumaran, and Subash Babu. 2022. "Multi-Dimensionality Immunophenotyping Analyses of MAIT Cells Expressing Th1/Th17 Cytokines and Cytotoxic Markers in Latent Tuberculosis Diabetes Comorbidity" Pathogens 11, no. 1: 87. https://doi.org/10.3390/pathogens11010087